
)�������ڹ�ҵ�������й㷺����;��
��1��SO2�����ڹ�ҵ����SO3��
����һ�������£�ÿ����8g SO3���壬����9.83kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ__________________
����500�棬�������ڵ������£����ݻ�Ϊ1L�ļס��������ܱ������о�����2 mol SO2��1 mol O2���ױ���ѹǿ���䣬�ұ����ݻ����䣬��ַ�Ӧ����ﵽƽ�⡣
I��ƽ��ʱ����������SO3��������Ĺ�ϵΪ����_______�ң��>������<���� =������
II��������t1 minʱ�ﵽƽ�⣬��ʱ�����������SO2��ת����Ϊ90%����÷�Ӧ��ƽ�ⳣ��Ϊ_______�������¶Ȳ��䣬t2 minʱ����������г���1 mol SO2��1 mol SO3��t3 minʱ�ﵽ��ƽ�⡣������ͼ�л���t2~t4min�����淴Ӧ���ʵı仯���ߣ������ϱ������V���� V����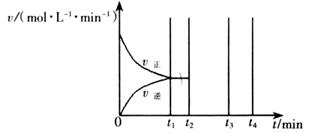
��2������þ���壨MgSO4��7H2O )���Ƹҽҩ��������й㷺��;��4.92g����þ����������ˮ���̵��������ߣ������������¶ȱ仯�����ߣ�����ͼ��ʾ��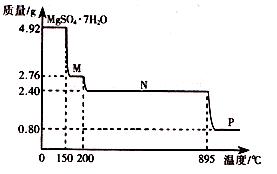
�ٹ���M�Ļ�ѧʽΪ__________��
������þ��������ʧȥ�ᾧˮ�Ĺ��̷�Ϊ_________���Ρ�
��Nת����Pʱ��ͬʱ������һ��������÷�Ӧ�Ļ�ѧ����ʽΪ_________��
��1���� 2SO2(g) + O2(g) =" 2" SO3(g) ��H =" -196.6" kJ/mol
��I���� II��810 L/mol 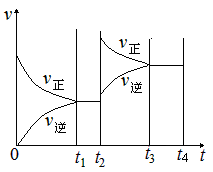
��2���� MgSO4��H2O �� 2 �� MgSO4 MgO+SO3��
MgO+SO3��
���������������1����д����ѧ����ʽ����Ӧ��Ϊ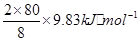 =196.6kJ��mol-1��
=196.6kJ��mol-1��
��I������SO3�ķ�Ӧ�����������С�ķ�Ӧ����ѹ�����൱���ں��������Ļ�����������ѹǿ����˼�ת���ʸߣ����ɵ�SO3�ࡣ
II���������⣬�� 2SO2 + O2 =" 2" SO3
��ʼ��/mol�� 2 1 0
�仯��/mol�� 2��90% 1��90% 2��90%
ƽ����/mol�� 0.2 0.1 1.8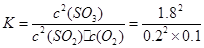 =810
=810
�ڸ÷�Ӧ�����£�����1molSO3����ԭƽ���ǵ�Чƽ�⣬����1molSO2��ƽ�������ƶ��������ͼʱע�⣬ƽ�������ƶ�������Ӧ���ʴ����淴Ӧ���ʣ�����ԭƽ��Ļ����ϸ����ʵ�Ũ�ȶ�����
��2����4.92g MgSO4��7H2O�����ʵ���Ϊ0.02mol����M����Է�������Ϊ �����M�ķ���ʽΪMgSO4��H2O��
�����M�ķ���ʽΪMgSO4��H2O��
�ڸ��ݢٿ�֪����һ��ʧȥ6���ᾧˮ����ʣ��1����ֻ��Ҫһ���Σ���˹���2���Ρ�
�۸������⣬M��Nʧȥ���нᾧˮ��N������ΪMgSO4��������ΪMgSO4�ֽ⣬�����������ݣ�Mg�����ʵ���Ϊ0.02mol����P�ε����ʵ���Է�������Ϊ0.8g��0.02mol=40g/mol�����ֽ����ΪMgO������ԭ���غ㣬��һ������ΪSO3��
���㣺�����Ȼ�ѧ����ʽ��д����Чƽ�⣬ƽ�ⳣ�����㣬Ӱ�컯ѧƽ������أ���ѧƽ��ͼ��ͼ�������ʵ�����ݴ����ȡ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ǵ����Ϻ����ḻ��һ��Ԫ�أ���Ԫ�صĵ��ʺͻ������ڹ�ũҵ����������������Ҫ��;��
��1���������������仯ʾ��ͼ��
д��CO��NO2��Ӧ����NO��CO2���Ȼ�ѧ����ʽ
��2���ڹ̶�������ܱ������У��������»�ѧ��Ӧ��N2(g)+3H2(g)  2NH3 (g) ��H<0��
2NH3 (g) ��H<0��
��ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±������ж�K1 K2���>������=����<����
| T /K | 298 | 398 |
| ƽ�ⳣ��K | K1 | K2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��15�֣�
�״���һ�ֿ�������Դ�����й㷺�Ŀ�����Ӧ��ǰ������ش�������״��йص����⣮
��1���״�������____���ӣ�����ԡ��Ǽ��ԡ�����
��2����ҵ��һ��ɲ������·�Ӧ���ϳɼ״���CO(g)+2H2(g) CH3OH(g)��H=-86.6kJ/mol����T��ʱ����һ������̶�Ϊ1L���ܱ������м���1mol CO��2mol H2������Ӧ�ﵽƽ��ʱ�������ڵ�ѹǿ�ǿ�ʼʱ��3/5��
CH3OH(g)��H=-86.6kJ/mol����T��ʱ����һ������̶�Ϊ1L���ܱ������м���1mol CO��2mol H2������Ӧ�ﵽƽ��ʱ�������ڵ�ѹǿ�ǿ�ʼʱ��3/5��
�ٴﵽƽ��ʱ��CO��ת����Ϊ ��
������ѡ�����жϸ÷�Ӧ�ﵽƽ��״̬�����ݵ���____ ��
A�� | B��CO���������ʵ���CH3OH���������� |
| C�������ڵ�ѹǿ���ֲ��� | D�����������ܶȱ��ֲ��� |
 ��3����֪�ڳ��³�ѹ�£�
��3����֪�ڳ��³�ѹ�£�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
̼��̼�Ļ������������������е�Ӧ�÷dz��㷺�����ᳫ���������ѳɳ����Ľ��죬����̼�������ֻ��һ�����룬����һ��ֵ���ڴ����µ����ʽ��
��1����CO2�뽹̿��������CO��CO�����������ȡ�
����֪��Fe2O3(s)��3C(ʯī)��2Fe(s)��3CO(g) ��H1�� +489.0 kJ/mol
C(ʯī)��CO2(g)��2CO(g) ��H2��+172.5 kJ/mol
��CO��ԭFe2O3���Ȼ�ѧ����ʽΪ ��
���Ȼ��٣�PdCl2����Һ����Ӧ���ڼ���������CO��PdCl2����ԭ�ɵ��ʣ���Ӧ�Ļ�ѧ����ʽΪ ��
��2��������ʯī�缫����KOH��Һ�У��������ֱ�ͨ��C3H8��O2���ɱ���ȼ�ϵ�ء�
�ٸ����缫��Ӧʽ�ǣ� ��
��ijͬѧ���ñ���ȼ�ϵ�������һ�ֵ�ⷨ��ȡFe(OH)2��ʵ��װ�ã�����ͼ��ʾ����ͨ�����Һ�в��������İ�ɫ�������ҽϳ�ʱ�䲻��ɫ������˵������ȷ���� ������ţ�
A����Դ�е�aһ��Ϊ������bһ��Ϊ����
B��������NaCl��Һ��Ϊ���Һ
C��A��B���˶������������缫
D�����������ķ�Ӧ�ǣ�2H+��2e����H2��
��3������ͬ����CO(g)��H2O(g)�ֱ�ͨ�����Ϊ2L�ĺ����ܱ������У����з�Ӧ��CO(g)��H2O(g)  CO2(g)��H2(g)���õ������������ݣ�
CO2(g)��H2(g)���õ������������ݣ�
| ʵ���� | �¶�/�� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | |
| H2O | CO | CO2 | |||
| 1 | 650 | 2 | 4 | 1.6 | 5 |
| 2 | 900 | 1 | 2 | 0.4 | 3 |
| 3 | 900 | 1 | 2 | 0.4 | 1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
Ϊ��СCO2�Ի�����Ӱ�죬���������ŷ�����ͬʱ��Ӧ��ǿ��CO2�������õ��о���
��1���ٰѺ��нϸ�Ũ��CO2�Ŀ���ͨ�뱥��K2CO3��Һ�����ڢٵ�����Һ��ͨ����ˮ�����õ���Ũ�ȵ�CO2���塣д�����з�Ӧ�Ļ�ѧ����ʽ__________________________��
��2���罫CO2��H2��1:3������Ȼ�ϡ�
���ʵ������ºϳ�ij����ˮ�������� ������ţ���
| A������ | B��ϩ�� | C��Ȳ�� | D������ͬϵ�� |
 CH3OH(g)+H2O(g) ��H=��49.0 kJ/mol�����CO2(g)��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
CH3OH(g)+H2O(g) ��H=��49.0 kJ/mol�����CO2(g)��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
| �ܽ�ȣ�S��/g | �ܶȻ���Ksp�� | ||
| Ca(OH)2 | Ba(OH)2 | CaCO3 | BaCO3 |
| 0.16 | 3.89 | 2.9��10��9 | 2.6��10��9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
I����¯������ұ��������Ҫ��������������Ҫ��ӦΪ��
Fe2O3(s) + 3CO(g) 2Fe(s)+3CO2(g) ��H
2Fe(s)+3CO2(g) ��H
��1����֪����Fe2O3(s) + 3C(ʯī)="2Fe(s)" + 3CO(g) ��H1
��C(ʯī��+ CO2(g) = 2CO(g) ��H2
���H___________________(�ú���H1 ����H2�Ĵ���ʽ��ʾ)��
��2����¯������Ӧ��ƽ�ⳣ������ʽK=____________________________��
��3����ij�¶�ʱ���÷�Ӧ��ƽ�ⳣ��K=64����2L�����ܱ����������У��ֱ��±���ʾ�������ʣ���Ӧ����һ��ʱ���ﵽƽ�⡣
| | Fe2O3 | CO | Fe | CO2 |
| ��/mol | 1.0 | 1.0 | 1.0 | 1.0 |
| ��/mol | 1.0 | 1.5 | 1.0 | 1.0 |
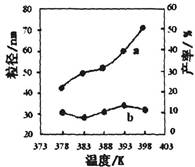
| ��� | �¶�/K | ��Ӧʱ��/h | ��Ӧ�����ʵ������ | ʵ��Ŀ�� |
| �� | 378 | 4 | 3��1 | ʵ��ں͢�̽��________ ______________________ ʵ��ں�__________̽�� ��Ӧʱ��Բ��ʵ�Ӱ�졣 |
| �� | 378 | 4 | 4��1 | |
| �� | 378 | 3 | _______ | |
| �� | 398 | 4 | 4��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��ȥ���ʺ��ˮú����Ҫ��H2��CO��������ĺϳɼ״���ԭ������
��1������ˮú�������������·�Ӧ����C(s)+CO2(g) 2CO(g) ��H1��
2CO(g) ��H1��
��CO(g)+H2O(g) H2(g)+CO2(g) ��H2����C(s)+H2O(g)
H2(g)+CO2(g) ��H2����C(s)+H2O(g) CO(g)+H2(g) ��H3��
CO(g)+H2(g) ��H3��
������Ӧ��H3���H1����H2֮��Ĺ�ϵΪ ��
��2����CH4ת����CO����ҵ�ϳ����ô�ת���������䷴Ӧԭ��Ϊ��2CH4(g)��3O2(g) 4CO(g)��4H2O(g) ��H=��1038kJ/mol����ҵ��Ҫѡ����ʵĴ������ֱ��X��Y��Z���ִ�����������ʵ�飨����������ͬ����
4CO(g)��4H2O(g) ��H=��1038kJ/mol����ҵ��Ҫѡ����ʵĴ������ֱ��X��Y��Z���ִ�����������ʵ�飨����������ͬ����
��X��750��ʱ��Ч����ߣ���ʹ����Ӧ���ʼӿ�Լ3��105����
��Y��600��ʱ��Ч����ߣ���ʹ����Ӧ���ʼӿ�Լ3��105����
��Z��440��ʱ��Ч����ߣ���ʹ�淴Ӧ���ʼӿ�Լ1��106����
����������Ϣ������Ϊ��������Ӧ��ѡ������˴����� ���X����Y����Z������ѡ��������� ��
��3�����ڴ���У�������2���з�Ӧ���д�����������������·�Ӧ��������ϵ�����仯ʾ��ͼ�������б�Ҫ��ע��
��4���ϳ����ϳɼ״�����Ҫ��Ӧ�ǣ�2H2(g)+CO(g) CH3OH(g) ��H=��90.8kJ��mol��1��T���´˷�Ӧ��ƽ�ⳣ��Ϊ160��
CH3OH(g) ��H=��90.8kJ��mol��1��T���´˷�Ӧ��ƽ�ⳣ��Ϊ160��
���¶��£����ܱ������п�ʼֻ����CO��H2����Ӧ10min���ø���ֵ�Ũ�����£�
| ���� | H2 | CO | CH3OH |
| Ũ��/��mol��L��1�� | 0.20 | 0.10 | 0.40 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijʵ��С����0.5mol/LNaOH��Һ��0.5mol/L������Һ��Ӧ�����к��ȵIJⶨ��
��.����0.5mol/LNaOH��Һ��
��1����ʵ���д�ԼҪʹ��245mlNaOH��Һ��������Ҫ����NaOH���� g��
��2��������������ѡ�����NaOH�������������������ĸ�� ��
a��������ƽ�������룩b.СֽƬc.С�ձ�d.����ǯ e.������ f.ҩ�� g.��Ͳ
��.�ⶨϡ�����ϡ���������к��ȵ�ʵ��װ����ͼ��ʾ��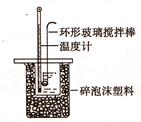
��1��д���÷�Ӧ�����к��ȱ�ʾ���Ȼ�ѧ����ʽ(�к���Ϊ57.3kJ/mol) ��
(2)ȡ50mlNaOH��Һ��30ml������Һ����ʵ�飬ʵ�����������ʾ��
������д���еĿհף�
| �¶� ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶� t2/�� | �¶Ȳ�ƽ��ֵ (t2-t1)/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 30.1 | |
| 2 | 27.0 | 27.4 | 27.2 | 33.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.8 | |
| 4 | 26.4 | 26.2 | 26.3 | 30.4 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
һ���������ܱ������з������·�Ӧ��N2(g)+3H2(g)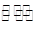 2NH3(g)����˵���÷�Ӧ�ﵽ��ѧƽ��״̬���� �� ��
2NH3(g)����˵���÷�Ӧ�ﵽ��ѧƽ��״̬���� �� ��
| A��N2��H2��NH3��Ũ����� |
| B��N2��H2��NH3��Ũ�Ȳ��ٱ仯 |
| C��N2��H2��NH3���ܱ������й��� |
| D����Ӧֹͣ�������淴Ӧ���ʶ������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com