
| ����������Һ |
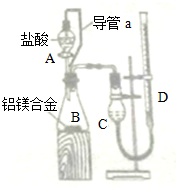
| ���� |
| m1-m2 |
| m1 |
| m1-m2 |
| m1 |


 ������״Ԫ���Ծ�ϵ�д�
������״Ԫ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
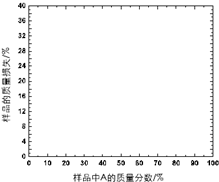 ������A��һ�����ȶ��Խϲ����ˮ���������Σ������·���������з�������A��������ϻ�Ͼ����Ƴ���Ʒ��������400�棬��¼��A����ͬ����Ʒ��������ʧ��%������������±�������������Ϣ���±������ݣ�ͨ����ͼ���ƶϻ�����A�Ļ�ѧʽ����������Ҫ������̣�
������A��һ�����ȶ��Խϲ����ˮ���������Σ������·���������з�������A��������ϻ�Ͼ����Ƴ���Ʒ��������400�棬��¼��A����ͬ����Ʒ��������ʧ��%������������±�������������Ϣ���±������ݣ�ͨ����ͼ���ƶϻ�����A�Ļ�ѧʽ����������Ҫ������̣�| ��Ʒ��A����������/% | ��Ʒ��������ʧ/% |
| 20 | 7.4 |
| 50 | 18.5 |
| 70 | 25.8 |
| 90 | 33.3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
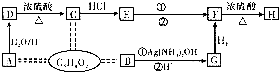
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��HNO3 |
| B��C2H5OH |
| C��NH4NO3 |
| D��SO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ڻ�����м������ᣬ��������÷�Һ©������ |
| B���ڻ�����м���NaOH��Һ���������ͨ�����CO2���壬����ȫ��Ӧ���÷�Һ©������ |
| C������������������� |
| D���ڻ�����м������ѣ��������ȡ���ӣ�Ȼ�����÷�Һ©������ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com