
��ǰ����������һ��ȫ�����ӵ����⣬������������峣��������������CO2��
��Ⱦ������NOx��SOx�ȡ��������Щ����������þͿ��Գ�Ϊ��Ҫ����Դ���Ƚ���˶Ի�������Ⱦ���ֽ���˲�����ԴΣ�����⡣
(1)������̼�ǵ�������ЧӦ��������ף�Ŀǰ���Ǵ���������̼�ķ���֮һ��ʹ����������Ӧ�ϳɼ״����״�������ȼ�ϵ�ص���Ҫȼ�ϡ�CO2��H2��Ӧ�Ʊ�CH3OH��H2O�Ļ�ѧ����ʽΪ
(2)�ڸ�����һ����̼�ɽ���������ԭΪ������
��֪��
��C(s)��O2(g)=CO2(g)��H1����393.5 kJ��mol��1
��CO2(g)��C(s)=2CO(g)��H2����172.5 kJ��mol��1
��S(s)��O2(g)=SO2(g)��H3����296.0 kJ��mol��1
��д��CO��SO2��Ӧ���Ȼ�ѧ����ʽ ��
(3)���᳧���ô���ԭ��������β����CH4�ڴ������¿��Խ�NO2��ԭΪN2��
��֪��CH4(g)��2O2(g)=CO2(g)��2H2O(g)��H����889.6 kJ��mol��1��
N2(g)��2O2(g)=2NO2(g)��H����67.7 kJ��mol��1��
��CH4��ԭNO2����ˮ�����͵������Ȼ�ѧ����ʽ�� ��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ�Ǻ���P��s����Cl2��Ӧ����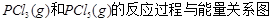 ��ͼ�е�
��ͼ�е� ��ʾ����1mol��������ݣ���������ͼ�ش��������⣺
��ʾ����1mol��������ݣ���������ͼ�ش��������⣺
��1�� ���Ȼ�ѧ����ʽΪ ��
���Ȼ�ѧ����ʽΪ ��
��2��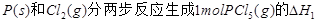 = KJ��mol-1
= KJ��mol-1
��3���о���������ѧ��Ӧ�������仯����H���뷴Ӧ���������ļ����йء����ܿ��Լ�����Ϊ�Ͽ�1mol��ѧ��ʱ�������յ���������1�����Dz��ֻ�ѧ���ļ������ݡ�
��1���ֻ�ѧ���ļ�������
| ��ѧ�� | P-P | P-O | O=O | P=O |
| ����/��kJ��mol-1�� | 198 | 360 | 498 | x |
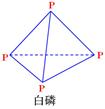 ��P4O10��
��P4O10��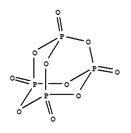
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ҵ����ͺ�������35%,ʹȼ��ȼ�ո��ӳ��,ʹ�ó����Ҵ����ͣ�β���ŷŵ�CO��̼�⻯����ƽ������30%����,��Ч�Ľ��ͺͼ������к���β���ŷš���������ʹ���Ҵ����Ͳ����ܼ���NOx���ŷţ���NOx����Ч������Ϊ�����������Ҫ���⡣NOx��������У��γ����꣬��ɿ�����Ⱦ��NOx����һ�ֺ���ɫ���壬������ˮ�ķ���ʽ�� ��
��2����֪NO2��N2O4�Ľṹʽ�ֱ���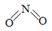 ��
��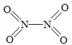 ��
��
| ���� | NO2 | N2O4 | |
| ��ѧ�� | N��O | N��N | N��O |
| ���ܣ�kJ/mol�� | 466 | 167 | 438 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
̼�͵��Ļ���������������������������ء�
��1����һ���¡������ܱ������з�����Ӧ�� Ni(s)+4CO(g) Ni(CO)4(g)��
Ni(CO)4(g)�� H<0�����ø÷�Ӧ���Խ�����ת��Ϊ���ȴ�99��9���ĸߴ������Ը÷�Ӧ��˵����ȷ���� (����ĸ���)��
H<0�����ø÷�Ӧ���Խ�����ת��Ϊ���ȴ�99��9���ĸߴ������Ը÷�Ӧ��˵����ȷ���� (����ĸ���)��
| A������Ni���������CO��ת���ʣ�Ni��ת���ʽ��� |
B����С�����ݻ���ƽ�����ƣ� H��С H��С |
| C����Ӧ�ﵽƽ�����CO�ٴδﵽƽ��ʱ��CO������������� |
| D����4v[Ni(CO)4]=v(CO)ʱ�������л�������ܶȲ���ʱ������˵����Ӧ�Ѵﻯѧƽ��״̬ |
 O2(g)=CO(g)
O2(g)=CO(g)  H=-Q1 kJ
H=-Q1 kJ mol-1
mol-1 H=-Q2 kJ
H=-Q2 kJ mol-1
mol-1 H=-Q3 kJ
H=-Q3 kJ mol-1
mol-1 H= ��
H= ��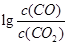 ���¶ȣ�t���Ĺ�ϵ����ͼ��
���¶ȣ�t���Ĺ�ϵ����ͼ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������״������ʵ����ȼ�ϣ�������ȼ�ϵ�ء�
��1����֪����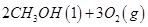 =
=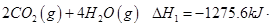 mol
mol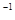
�� =
=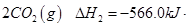 mol
mol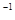
��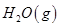 =
=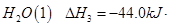 mol
mol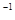
д���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ ��
��2�������״���ԭ��CO��H2��Դ�����з�Ӧ��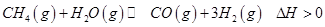
��һ�������� ��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼa����
��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼa����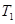
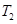 (�<������>����="����ͬ)��A��B��C���㴦��Ӧƽ�ⳣ��(
(�<������>����="����ͬ)��A��B��C���㴦��Ӧƽ�ⳣ��(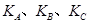 )�Ĵ�С��ϵΪ ��
)�Ĵ�С��ϵΪ ��
��100��ʱ����1 mol 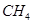 ��2 mol
��2 mol  ͨ���ݻ�Ϊ1L�Ķ����ܱ������з�����Ӧ����˵���÷�Ӧ�Ѿ��ﵽƽ��״̬���� (�����)��
ͨ���ݻ�Ϊ1L�Ķ����ܱ������з�����Ӧ����˵���÷�Ӧ�Ѿ��ﵽƽ��״̬���� (�����)��
a��������ѹǿ�㶨
b����λʱ��������0.1 mol CH4ͬʱ����0.3 molH2
c�������������ܶȺ㶨
d��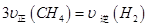
����ﵽƽ��ʱ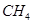 ��ת����Ϊ0��5����100��ʱ�÷�Ӧ��ƽ�ⳣ��K= ��
��ת����Ϊ0��5����100��ʱ�÷�Ӧ��ƽ�ⳣ��K= ��
��3��ijʵ��С������CO(g)�� ��KOH(aq)��Ƴ���ͼb��ʾ�ĵ��װ�ã���õ�ظ����ĵ缫��ӦʽΪ ��
��KOH(aq)��Ƴ���ͼb��ʾ�ĵ��װ�ã���õ�ظ����ĵ缫��ӦʽΪ ��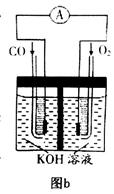
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ĵ��зḻ����Ȼ����Դ������Ȼ��Ϊԭ�Ϻϳ����ص���Ҫ��������ͼ��ʾ(ͼ��ijЩת�����輰������δ�г�)��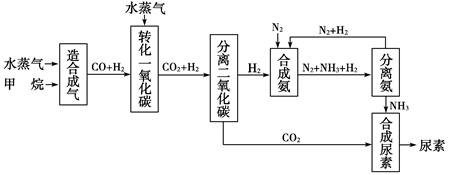
����д���пհף�
(1)��֪0.5 mol������0.5 molˮ������t �桢p kPaʱ����ȫ��Ӧ����һ��
��̼������(�ϳ���)��������a kJ�������÷�Ӧ���Ȼ�ѧ����ʽ�ǣ�______________________��
(2)�ںϳɰ���ʵ�����������У�����ȡ�Ĵ�ʩ֮һ�ǣ������ɵİ��ӻ�������м�ʱ����������������������ĵ���������ѭ�����ã�ͬʱ���䵪���������������û�ѧ��Ӧ���ʺͻ�ѧƽ��Ĺ۵�˵����ȡ�ô�ʩ�����ɣ�
________________________________________________________________��
(3)������ϳɰ�����ת����Ϊ75%ʱ����5.60��107 L����Ϊԭ���ܹ��ϳ�________L������(����������ڱ�״���²ⶨ)
(4)��֪���صĽṹ��ʽΪ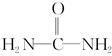 ����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ��
����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ��
��__________________�� ��_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
NOx������β���е���Ҫ��Ⱦ��֮һ��
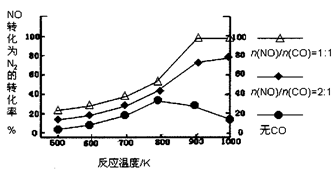
(1)NOx���γ����꣬д��NO2ת��ΪHNO3�Ļ�ѧ����ʽ��__________________________��
(2)��������������ʱ������N2��O2��Ӧ���������仯ʾ��ͼ���£�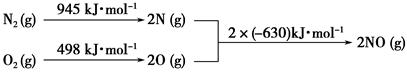
��д���÷�Ӧ���Ȼ�ѧ����ʽ��_______________________________��
�����¶����ߣ��÷�Ӧ��ѧƽ�ⳣ���ı仯������____��
(3)������β��ϵͳ��װ�ô�ת����������Ч����NOx���ŷš�
�ٵ�β���п�������ʱ��NOx�ڴ�ת�����б���ԭ��N2�ų���д��NO��CO��ԭ�Ļ�ѧ����ʽ��______________________________
�ڵ�β���п�������ʱ����ת�����еĽ�������������NOx�����Ρ�����������˳�����£�12MgO��20CaO��38SrO��56BaO��ԭ����___________________________________________��
Ԫ�صĽ���������ǿ�������������NOx��������������ǿ��
(4)ͨ��NOx�������ɼ��NOx�ĺ������乤��ԭ��ʾ��ͼ���£�
��Pt�缫�Ϸ�������________��Ӧ(���������ԭ��)
��д��NiO�缫�ĵ缫��Ӧʽ��______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ѣ�CH3OCH3����һ����Ҫ�ľ�ϸ������Ʒ������Ϊ�Ƕ�ʮһ��������DZ����ȼ��[ ��֪��CH3OCH3(g)+3O2(g)��2CO2(g)+3H2O��1�� ��H����1455kJ/mol ]��ͬʱ��Ҳ������Ϊ�������������ȴ�������ҵ���Ʊ������ѵ���Ҫ���������������Σ�
�ټ״�Һ����Ũ���������»�״������ڴ�������ֱ����ˮ�ƶ����ѣ� 2CH3OH 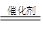 CH3OCH3��H2O
CH3OCH3��H2O
�ںϳ���CO��H2ֱ�Ӻϳɶ����ѣ� 3H2(g)��3CO(g) CH3OCH3(g)��CO2(g) ��H����247kJ/mol
CH3OCH3(g)��CO2(g) ��H����247kJ/mol
����Ȼ����ˮ������Ӧ�Ʊ������ѡ���CH4��H2OΪԭ���Ʊ������Ѻͼ״���ҵ�������£�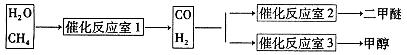
��1��д��CO(g)��H2(g)��O2(g)��Ӧ����CO2(g)��H2O��1�����Ȼ�ѧ����ʽ���������һλС����
��2���ڷ�Ӧ��2�У�һ�������·�����Ӧ3H2(g)��3CO(g) CH3OCH3(g)��CO2(g)���ܱ������дﵽƽ���Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ��
CH3OCH3(g)��CO2(g)���ܱ������дﵽƽ���Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ��
A�����¸�ѹ B���Ӵ��� C������COŨ�� D�������������
��3���ڷ�Ӧ��3�У���һ���¶Ⱥ�ѹǿ�����·����˷�Ӧ��3H2(g)��CO2(g)  CH3OH(g)��H2O (g) ��H��0��Ӧ�ﵽƽ��ʱ���ı��¶ȣ�T����ѹǿ��P������Ӧ�����CH3OH�����ʵ����������仯�����ͼ��ʾ�������¶ȣ�T����ѹǿ��P���Ĺ�ϵ�ж���ȷ���� ������ţ�
CH3OH(g)��H2O (g) ��H��0��Ӧ�ﵽƽ��ʱ���ı��¶ȣ�T����ѹǿ��P������Ӧ�����CH3OH�����ʵ����������仯�����ͼ��ʾ�������¶ȣ�T����ѹǿ��P���Ĺ�ϵ�ж���ȷ���� ������ţ�
A��P3��P2 T3��T2 B��P2��P4 T4��T2
C��P1��P3 T1��T3 D��P1��P4 T2��T3
��4����Ӧ��1�з�����Ӧ��CH4(g)��H2O(g) CO(g)��3H2(g) ��H��0д��ƽ�ⳣ���ı���ʽ��
CO(g)��3H2(g) ��H��0д��ƽ�ⳣ���ı���ʽ��
����¶Ƚ��ͣ��÷�Ӧ��ƽ�ⳣ�� ������䡱�����������С����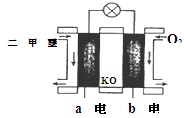
��5����ͼΪ��ɫ��Դ��������ȼ�ϵ�ء��Ĺ���ԭ��ʾ��ͼ��a�缫�ķ�ӦʽΪ��________________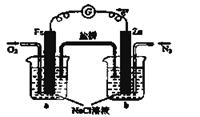
��6�������ж�����ȷ����_______
A�����ձ�a�м�������K3[Fe(CN)6]��Һ������ɫ��������
B���ձ�b�з�����ӦΪ2Zn-4e�� ��2Zn2+
C�����Ӵ�Zn������������Fe���������Żص�Zn��
D���ձ�a�з�����ӦO2 + 4H++ 4e�� �� 2H2O����ҺpH����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���̼�Ȼ�ԭ���Ȼ�����ʵ�����������Ʊ�������,����ط�Ӧ���Ȼ�ѧ����ʽ����:
Al2O3(s)+AlCl3(g)+3C(s) 3AlCl(g)+3CO(g)����H="a" kJ��mol-1
3AlCl(g)+3CO(g)����H="a" kJ��mol-1
3AlCl(g) 2Al(l)+AlCl3(g)����H="b" kJ��mol-1
2Al(l)+AlCl3(g)����H="b" kJ��mol-1
��ӦAl2O3(s) +3C(s) 2Al(l)+3CO(g)�Ħ�H=�������� kJ��mol-1(�ú�a��b�Ĵ���ʽ��ʾ)��
2Al(l)+3CO(g)�Ħ�H=�������� kJ��mol-1(�ú�a��b�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com