
����Ŀ����ҵ��ˮ�г�����һ������Cr2O72-��CrO42-�����ǻ�����༰��̬ϵͳ�����ܴ���˺���������д��������õĴ��������ǻ�ԭ���������÷��Ĺ�������Ϊ��![]()
���е���������ƽ�⣺2 CrO42-(��ɫ)+2H+![]() Cr2O72- (��ɫ)+H2O
Cr2O72- (��ɫ)+H2O
��1�� ��ƽ����ϵ��pH=12������Һ��_______ɫ��
��2�� ��˵����������Ӧ��ƽ��״̬����______________��
a��Cr2O72-��CrO42-��Ũ�Ȳ���
B��2v(Cr2O72-) =v(CrO42-)
C����Һ����ɫ����
��3���������У���ԭlmol Cr2O72-���ӣ���Ҫ_______mol��FeSO47H2O��
��4�����������ɵ�Cr(OH)3����Һ�д������³����ܽ�ƽ�⣺
Cr (OH)3 (s)![]() Cr3+ (aq) +3OH- (aq)
Cr3+ (aq) +3OH- (aq)
�����£�Cr(OH)3���ܶȻ�Ksp=c(Cr3+)��c3(OH-) = 10-32��Ҫʹ0.01mol/Lc(Cr3+)��ʼ��������Һ��pHӦ����__________����ʹc(Cr3+)����10-5mol/L����Һ��pHӦ����___________��
���𰸡���1������2��ac��3��6��4��4��5
��������
�����������1��c(OH-)����ƽ��2CrO42-(��ɫ)+2H+![]() Cr2O72-(��ɫ)+H2O���ƣ���Һ�ʻ�ɫ��
Cr2O72-(��ɫ)+H2O���ƣ���Һ�ʻ�ɫ��
��2�������ж�ƽ��״̬�ķ�����V��=V���������ֵ�Ũ�ȱ��ֲ�����˵���Ѵ�ƽ�⣻a��Cr2O72-��CrO42-��Ũ�Ȳ��ٸı䣬���ж�ƽ�⣬��a��ȷ��b��2v(Cr2O72-)=v(CrO42-)�������ж����淴Ӧ���ʹ�ϵ�������ж�ƽ�⣬��b����c����Һ����ɫ���䣬Ϊ�����������ж�ƽ�⣬��c��ȷ���ʴ�Ϊac��
��3�����ݵ��ӵ�ʧ�غ㣺n(Cr2O72-)��6=n(FeSO47H2O)��1��n(FeSO47H2O)=![]() =6mol��
=6mol��
��4��Ҫʹ0.01mol/Lc(Cr3+)��ʼ��������Һ��c(OH-)=![]() =10-10mol/L����ʱ��ҺpH=4����c(Cr3+)=10-5mol/Lʱ����Һ��c(OH-)=
=10-10mol/L����ʱ��ҺpH=4����c(Cr3+)=10-5mol/Lʱ����Һ��c(OH-)=![]() =10-9mol/L��c(H+)�T
=10-9mol/L��c(H+)�T![]() =10-5mol/L��pH=5����Ҫʹc(Cr3+)����10-5mol/L����Һ��pHӦ����5��
=10-5mol/L��pH=5����Ҫʹc(Cr3+)����10-5mol/L����Һ��pHӦ����5��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ����������ԭ��Ӧ����ˮ�Ȳ���������Ҳ������ԭ�������� ��
A��CO2+H2O=H2CO3 B��2F2+2H2O=4HF+O2
C��Cl2+H2O=HCl+HClO D��2Na+2H2O=2NaOH+H2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ����ϡ������п��Ӧ��ȡ������ʵ���У����ּ�����������ͭ��Һ���Լӿ��������������ʡ���ش��������⣺
��1�� ����ʵ���з�����Ӧ�Ļ�ѧ����ʽ�У�
__________________��_____________________��
��2�� ����ͭ��Һ���Լӿ������������ʵ�ԭ��__________________��
��3�� ʵ��������Na2SO4��MgSO4�� Ag2SO4�� K2SO4������Һ����������ʵ����CuSO4��Һ���������õ��ǣ�________��
��4�� Ҫ�ӿ�����ʵ����������������ʣ������Բ�ȡ�Ĵ�ʩ�У�
_____________________��___________________________�������֣���
��5�� Ϊ�˽�һ���о�����ͭ�����������������ʵ�Ӱ�죬��ͬѧ��������µ�һϵ�е�ʵ�顣�����������Ļ����Һ�ֱ�ӵ�6��ʢ�й���п���ķ�Ӧƿ�У��ռ����������壬��¼�����ͬ�����������Ҫ��ʱ�䡣
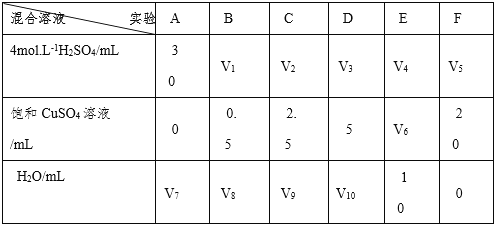
������ɴ�ʵ����ƣ����У�V1 =____��V6 = ____��V9=____��
�ڷ�Ӧһ��ʱ���ʵ��E�еĽ�����____ɫ��
�۸�ͬѧ���ó�����Ϊ����������������ͭ��Һʱ���������������ʻ�����ߣ��������������ͭ��Һ����һ����ʱ���������������ʷ����½���������������������½�����Ҫԭ��______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ɫ����һ���������ʽ��������Ϊ������ɫ��������������
A. ������չ�����Դ�����ٶԴ�ͳ��Դ������
B. �������������ռ�������ʵ����Դ��������
C. ��������������ʯ�ң��������ʴ������
D. �ƹ㹲��������������̼�������ij��з�ʽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪������Ϣ����1 mol N2�Ĺ��ۼ���������946 kJ��������1 mol H2�Ĺ��ۼ���������436 kJ���������γ�1 mol NH3�еĻ�ѧ���ͷ�1 173 kJ���������ڽ�һ������N2��H2Ͷ��һ�ܱ������У���һ�������½��з�Ӧ������й��������£�
N2(mol��L��1) | H2(mol��L��1) | NH3(mol��L��1) | |
��ʼʱ | 3 | 3 | 0 |
2sĩ | 2.6 | 1.8 | 0.8 |
��������������ݻش����⣺
(1)��H2��ʾ�÷�Ӧ2 s�ڵ�ƽ����Ӧ����Ϊ________
(2)______(��ܡ����ܡ�)ȷ�ϸ÷�Ӧ2 sĩ�Ѵﻯѧƽ��״̬��
(3)д���÷�Ӧ�Ļ�ѧ����ʽ��
________________________________________________________________________��
(4)�������������ɰ����Ĺ���______(��ͷš������ա�)������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ʷ����У�ǰ�߰������ߵ��ǣ� ��
A.�ǽ��������� ����������
B.������ �ǵ����
C.��Һ ����
D.����Һ ��ɢϵ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҩҩ����˪����Ҫ�ɷ�As2O3������ˮ����ҽ�����������Ƽ���Ѫ����ij��������һ�ֺ��龫��ʯ�ۣ���Ҫ�ɷ�ΪAs4S4��As2S3��FeS2�������������ʣ�Ϊԭ����ȡAs2O3, �������̼�ͼ���£�
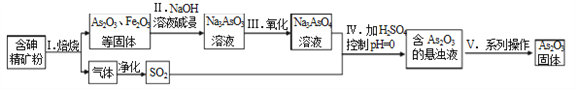
�ش��������⣺
(1)����I������SO2����ɻ��������ã������й�SO2��;��˵����ȷ����______��
A.��ҵ������ B.Ư��ֽ�� C.����ˮ����
(2)����II�з����ķ�Ӧ______������ԭ��Ӧ����ǡ����ǡ�)��
(3)����V��ϵ�в���Ϊ_______ (�ѧʵ������������ƣ���
(4)�ٹ���I�б���As2S3�Ļ�ѧ��Ӧ����ʽΪ_______________��
�ڹ���IV������As2O3�����ӷ�Ӧ����ʽΪ_______________��
(5)�ж���AsO33-ͨ����ⷴӦ��ת��Ϊ����AsO43-����ʯīΪ�缫����ǿ������Һ��
��⺬AsO33-����Һ�������ĵ缫��ӦʽΪ______________��
(6)�ⶨijAs2O3�ֲ�Ʒ����As2O5���ʣ���As2O3������������ʵ��������£�
a.��ȡm g�ֲ�Ʒ�ܽ���NaOH��Һ���õ���AsO33-��AsO43-�Ļ����Һl00mL��
b.�ֱ���ȡ25.00mL������Һ����0.02500 mol��L-1��I2����Һ���еζ���I2��AsO33-����ΪAsO43-��������ҺΪָʾ������ÿ�εζ���ʼʱҺ�������ͼһ��ʾ�����εζ�����ʱ��I2����ҺҺ�������ͼ��ͼ����ʾ��
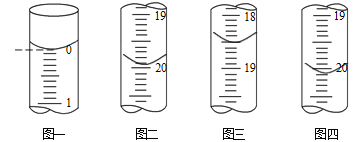
�������ζ��յ������_____________________��
�ڴֲ�Ʒ��As2O3����������Ϊ______________ (�ú���m�Ĵ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±���Ԫ�����ڱ���һ���֣��١������ijԪ�أ���ش��������⣺
���� ���� | IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 |
�� | �� | �� | �� | |||||
�� | �� | �� | �� | �� | �� | �� | ||
�� | �� |
��1���ڢ١�����,��ѧ��������õ�Ԫ����ԭ�ӽṹʾ��ͼΪ___________��
��2���ڢ١����У���������ǿ��Ԫ����_________(��Ԫ�ط���)���ڻ������о�ֻ�Ը��۵�Ԫ����__________(��Ԫ�ط���)��
��3���ڢܡ����У�Ԫ�ص�����������Ӧ��ˮ������������ǿ����__________(�����ʻ�ѧʽ,��ͬ)��������ǿ����________��
��4���ڢܡ����У�ԭ�Ӱ뾶��С����__________(��Ԫ�ط���)�������Ӱ뾶��С����_______(�����ӷ���)��
��5���ڢ١��ۡ��ߡ���Ԫ�ص���̬�⻯�������ȶ�����__________(���⻯�ﻯѧʽ)��
��6��д����ҵ���âٵ����ڸ���������ȡ�ߵ��ʵĻ�ѧ����ʽ______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�Ȼ��⣨�е�97.4�棩��һ�ֺ���ɫ�ӷ���Һ����������ˮ�������Ҵ������ᡣijУ�о���ѧϰС���ͬѧ���Ʊ�һ�Ȼ��⡣�ش��������⣺
��1������ͬѧ�����ø��������������ⷴӦ�Ʊ�һ�Ȼ��⣬��װ�����£�����֪���������ķ�ӦΪ���ȷ�Ӧ��
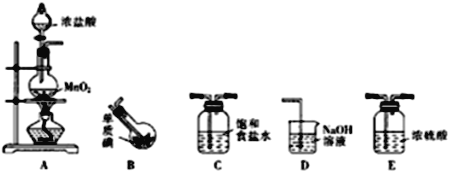
����װ������˳��ΪA��_________________��Aװ���з�����Ӧ�����ӷ���ʽΪ_____________________________��
��Bװ����ƿ�������ˮ�У���Ŀ����_____________________��Dװ�õ�������_________________��
����Bװ�õõ���Һ̬�����һ�ᴿ�ɵõ��ϴ�����ICl�����ᴿ��ȡ�IJ���������______________��
��2������ͬѧ���õ������±�������һ�Ȼ���ķ���������������ƿ�м���ֵ�����ᣬ�����¶�Լ50�����ڲ��Ͻ�������μ�����������Һ������һ�Ȼ��⡣������Ӧ�Ļ�ѧ����ʽΪ_______________________________��
��3�����ʵ��֤����
��ICl�������Ա�I2ǿ��__________________________��
��ICl����ϩ���÷����ӳɷ�Ӧ��_________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com