
| A������ͬ�����£�2C(ʯī) + O2(g) = 2CO(g) ��H =" -" 110.5 kJ��mol-1 |
| B��1 molʯī����ȫȼ�գ�����CO2��CO�������ʱ������504.0 kJ |
| C������ͬ�����£�C(ʯī) + CO2(g) = 2CO(g) ��H =" -" 172.5 kJ��mol-1 |
| D����֪���ʯȼ���ȴ���ʯī��ȼ���ȣ���ʯīת��Ϊ���ʯ��Ҫ���� |


 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
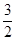 O2(g);��H="+765.2" kJ��mol-1�����ڴ��������ͼ�У�����������Ӧ��������ϵ�����仯ʾ��ͼ�������б�Ҫ��ע��
O2(g);��H="+765.2" kJ��mol-1�����ڴ��������ͼ�У�����������Ӧ��������ϵ�����仯ʾ��ͼ�������б�Ҫ��ע���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���������кͷ�Ӧ | B��ʳ�������������� |
| C������ʯ��ʯ���� | D��ըҩ��ը |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ��ѧ���� | H2(g) | Br2(g) | HBr(g) |
| 1mol�����еĻ�ѧ������ʱ��Ҫ���յ�����/KJ | 436 | a | 369 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A��;��II��ˮú��ʱ�����ܺģ���;��II����������ȡ |
| B����;��I��ȣ�;��II���Լ��ٶԻ�������Ⱦ |
| C����;��I��ȣ�;��II�������ú��ȼ��Ч�� |
| D����úת��Ϊˮú������ͨ���ܵ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A�����ʯ��ʯī�ȶ���ǿ |
| B��ʯī��Ϊ���ʯ�������仯 |
| C��ʯī��Ϊ���ʯ�Ƿ��ȷ�Ӧ |
| D��1 molʯī���ܼ��ܱ�1 mol���ʯ���ܼ��ܴ�1.9 kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Ƭ��ϡ���ᷴӦ | B��NH4Cl��Ba(OH)2��8H2O�ķ�Ӧ |
| C�����ȵ�̼������O2��ȼ�� | D������кͷ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������ʵ������������������ֱ���ȫȼ�գ�ǰ�߷ų������� |
| B���������оݼ����ж���NH4��2CO3���ȷֽ���Է����� |
| C���Ȼ�ѧ����ʽδע���¶Ⱥ�ѹǿ����H��ʾ��״���µ����� |
| D�����Է����еķ�Ӧһ���Ƿ��ȷ�Ӧ�������Է����еķ�Ӧһ�������ȷ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��4Q1��0.5Q2 | B��4Q1��Q2��10Q3 | C��4Q1��2Q2 | D��4Q1��0.5Q2��9Q3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com