
���� ��1����PV=nRT=$\frac{m}{M}$RT����֪PM=��RT�����¶ȡ��ܶ���ͬ�����£�ѹǿ����Է��������ɷ��ȣ�
��2����������������ȣ�ͬ��ͬѹ�£�����������ʵ�����ȣ�����������������̼����֮������������ʵ������ٽ�϶�����̼������������֮����������Ħ��������
��3����ͬ�����£�������ܶ�֮�ȵ�����Ħ������֮�ȣ����ɵĻ�����������������ܶ�ΪD�����������ƽ��Ħ������Ϊ2mg/mol�����������غ��ٽ��M=$\frac{m}{n}$����A��Ħ��������Ħ����������ֵ�ϵ�������Է���������
��� �⣺��1����PV=nRT=$\frac{m}{M}$RT����֪PM=��RT�����¶ȡ��ܶ���ͬ�����£�ѹǿ����Է��������ɷ��ȣ���P��CO����P��O2��=32��28=8��7����P��CO����P��O2����
�ʴ�Ϊ��P��CO����P��O2����
��2����������������ȣ�ͬ��ͬѹ�£�����������ʵ�����ȣ�����������ʵ���Ϊnmol����nmol����44g/mol-32g/mol��=122g-116g�����n=0.5
��������Ħ������ΪMg/mol����0.5mol����44g/mol-Mg/mol��=122g-114g�����M=28��
�ʸ��������Է�������Ϊ28���ʴ�Ϊ��28��
��3����ͬ�����£�������ܶ�֮�ȵ�����Ħ������֮�ȣ����ɵĻ�����������������ܶ�Ϊm�����������ƽ��Ħ������Ϊ2m/mol��������2molA��Ӧ������1molB��2molC��2molD�������������=2mg/mol����1+2+2��mol=10mg����Ӧǰ���������䣬��A������Ϊ10mg����Ħ������=$\frac{10mg}{2mol}$=5mg/mol��Ħ����������ֵ�ϵ�������Է�������������A����Է�������Ϊ5m������Ħ������Ϊ5mg/mol���ʴ�Ϊ��5mg/mol��
���� ���⿼���˰���٤�����ɼ������ۣ���ȷ��ͬ�����²�ͬ�����ܶ�������Է��������Ĺ�ϵ�ǽⱾ��ؼ����ٽ�ϻ�����ʽ���������Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ȡ������������茶�������Թܣ����ȣ��Թܿ���Һ�����ɣ����֤������ijɷ��к��нᾧˮ | |
| B�� | ��������茶�������ˮ���õ���ɫ��Һ������2��KSCN��Һ����Һ���Ժ�ɫ���ٵ��뼸��������ˮ����Һ��Ϊ��ɫ�����֤������ijɷ��к���Fe2+ | |
| C�� | ��������茶�������ˮ��������ϡ���ᣬ�������ٵ��뼸��BaCl2��Һ���а�ɫ�������ɣ����֤������ijɷ��к���SO42- | |
| D�� | ȡ������������茶�������Թܣ�����Ũ����������Һ�����ȣ��Թܿ�ʪ�����ɫʯ����ֽ��죬���֤������ijɷ��к���NH4+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
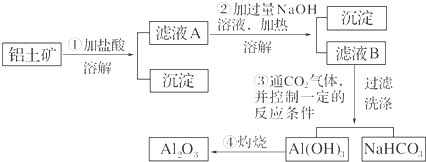
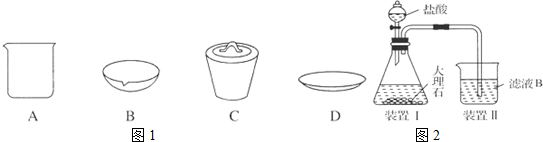
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ܢݢ� | B�� | �ڢۢܢ� | C�� | �٢ۢݢ� | D�� | �٢ڢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���� | B�� | CuSO4��Һ | C�� | NaOH��Һ | D�� | H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
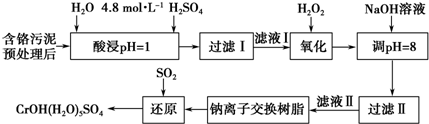
| ������ | Fe3+ | Mg2+ | Al3+ | Cr3+ |
| ��ʼ����ʱ��pH | 2.7 | - | - | - |
| ������ȫʱ��pH | 3.7 | 11.1 | 8 | 9����9�ܽ⣩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ϡ���ᷴӦ��2Fe+6H+�T2Fe3++3H2�� | |
| B�� | �ƺ���ˮ��Ӧ Na+2H2O�TNa++2OH-+H2�� | |
| C�� | ����������Һ��ϡ H2SO4 ��Ӧ��Ba2++2OH-+SO42-+2H+�TBaSO4��+2H2O | |
| D�� | ����NaHCO3��Һ��Ca��OH��2��Һ��Ӧ��OH-+HCO3-�TCO32-+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�߶��ϵ�һ���¿���ѧ���������棩 ���ͣ�ѡ����
ͼ�е����߱�ʾ������������һ��ʱ����Ӧ��2NO+02 2N02 ��H<0��N0��ƽ��ת�������¶ȵĹ�ϵ��ͼ�б���a��b��c��d�ĵ㣬���б�ʾδ�ﵽƽ��״̬����v(��)��v(��)�ĵ��� ( )
2N02 ��H<0��N0��ƽ��ת�������¶ȵĹ�ϵ��ͼ�б���a��b��c��d�ĵ㣬���б�ʾδ�ﵽƽ��״̬����v(��)��v(��)�ĵ��� ( )
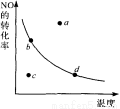
A��a�� B��b�� C. c�� D��d��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
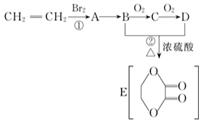 ����ϩ��������ԭ�Ϻϳɻ�״������ϳɹ������£�ˮ���������������ʡ�ԣ�
����ϩ��������ԭ�Ϻϳɻ�״������ϳɹ������£�ˮ���������������ʡ�ԣ� +2H2O��
+2H2O���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com