
��.ij��ɫ��ҺX����K+��![]() ��Ba2+��Al3+��Fe3+��
��Ba2+��Al3+��Fe3+��![]() ��
��![]() �е�������������ɣ�ȡ����Һ��������ʵ�飺
�е�������������ɣ�ȡ����Һ��������ʵ�飺
��1����ɫ�������� ��
��2����д��ʵ���������������A��B�����ӷ���ʽ �� ��
��3��ͨ������ʵ�飬��ȷ��X��Һ��һ�����ڵ������� ��Ҫȷ�����ܴ��ڵ����ӣ��貹�ӵ�ʵ���� ��
��.A��B��C����ǿ����ʣ�������ˮ�е�������������±���ʾ��

��ͼ��ʾװ���У��ס��ҡ��������ձ����ηֱ�ʢ��������A��Һ��������B��Һ��������C��Һ���缫��Ϊʯī�缫��
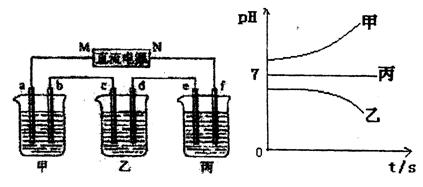
��ͨ��Դ������һ��ʱ��������c�缫����������16g�������¸��ձ�����Һ��pH����ʱ��t�Ĺ�ϵͼ���ϡ��ݴ˻ش��������⣺
��1��MΪ��Դ�� ������д�������������缫b�Ϸ����ĵ缫��ӦΪ ��
��2������缫e�����ɵ������ڱ�״���µ���� ��
��3��д�����ձ��ĵ��ط�Ӧ ��
��4�������������B��Һ�еĽ�������ȫ����������ʱ����ܷ�������У�Ϊʲô��
��5��������һ��ʱ��������c�缫����������16g��Ҫʹ���ָ���ԭ����״̬�������� ��
(16��)
�� ��1��BaSO4(1��)
��2��![]() +2H+=CO2��+H2O��1�֣�
+2H+=CO2��+H2O��1�֣� ![]() +OH-
+OH-![]() NH3��+H2O(1��)
NH3��+H2O(1��)
��3��![]() ��
��![]() ��
��![]() ��2�֣� ����ɫ��Ӧ������ɫ�ܲ���Ƭ���������ɫ��K+������K+����2�֣�
��2�֣� ����ɫ��Ӧ������ɫ�ܲ���Ƭ���������ɫ��K+������K+����2�֣�
��.��1������1�֣���4OH--4e-=2H2O+O2�� (1��)
��2��5.6L��1�֣�
��3��2CuSO4+2H2O![]() 2Cu+O2��+2H2SO4��2�֣�
2Cu+O2��+2H2SO4��2�֣�
��4���ܣ���ΪCuSO4��Һ��ת��ΪH2SO4��Һ����Ӧ��Ϊ���ˮ�ķ�Ӧ��2�֣�
��5������ձ��м�4.5gˮ��2�֣�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
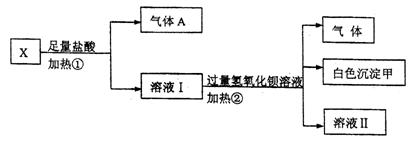
��14�֣�ij��ɫ��ҺX����K+��Ba2+��Al3+��Fe3+��AlO-2��CO2-3��SO2-4�е�������������ɣ�ȡ����Һ��������ʵ�飺
��1������A�� ���ѧʽ����ͬ��������B��
��2��д��������в�����������ӷ���ʽ____
��3���ֱ�д���γɰ�ɫ�����ס���������B�ķ�Ӧ�����ӷ���ʽ ��
��4��ͨ������ʵ�飬��ȷ��X��Һ��һ�����ڵ������� ��
��δȷ���Ƿ���ڵ������� �������һ���ĺ���ʵ����ȷ���������Ƿ���ڣ�����Ƶ�ʵ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
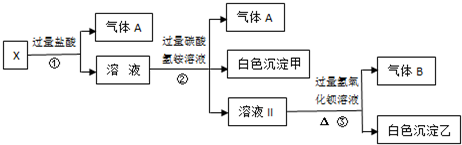
��8�֣�ij��ɫ��ҺX����K+��NH+4��Ba2+��Al3+��Fe3+��CO2-3��SO2-4�е�������������ɣ�ȡ����Һ��������ʵ�飺
��1����ɫ�������� ��
��2����д��ʵ���������������A��B�����ӷ���ʽ ��
��
��3��ͨ������ʵ�飬��ȷ��X��Һ��һ�����ڵ������� ����δȷ���Ƿ���ڵ������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com