


 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��2 L����������ֽ��1 L������1 L��������270 kJ���� |
| B��1 mol������1 mol������Ӧ����2 molҺ̬������ų�����С��270 kJ |
| C������ͬ�����£�1 mol������1 mol�����������ܺʹ���2 mol��������������� |
| D��1������������1���������ӷ�Ӧ����2��������������ӷų�270 kJ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����67.7 kJ��mol��1 | B����43.5 kJ��mol��1 |
| C����43.5 kJ��mol��1 | D����67.7 kJ��mol��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��H2 + O2 = 2H2O��H= -571.6KJ/mol |
| B��H2��g��+ 1/2O2��g��= H2O (l)��H= -142.9KJ/mol |
| C��H2��g��+ 1/2O2��g��= H2O (l)��H= -285.8KJ/mol |
| D��2H2��g��+ O2��g�� = 2H2O ��g����H=" -571.6KJ/mol" |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 ��
�� 

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��KOH(aq) + 1/2 H2SO4(aq) = 1/2K2SO4(aq) + H2O(l)����H ����11.46kJ/mol |
| B��2KOH(s) + H2SO4(aq) = K2SO4(aq) + 2H2O(l)����H ����114.6kJ/mol |
| C��2KOH(aq) + H2SO4 =K2SO4(aq) + H2O(l)����H ����114.6kJ/mol |
| D��KOH (aq) + 1/2 H2SO4(aq) = 1/2K2SO4(aq) + H2O(l)����H ����57.3kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2NH3�����ֱ���t��ʱ�ⶨ����NH3�������������ͼ���ң�
2NH3�����ֱ���t��ʱ�ⶨ����NH3�������������ͼ���ң�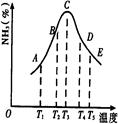
| A�������¶� | B�������¶� | C������ѹǿ | D����Сѹǿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com