
 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ©����
©�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 �����ᾧˮ��
�����ᾧˮ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��SO2ʹƷ����ɫ�Ļ�����ʹ����KMnO4��ɫ�Ļ���һ�� |
| B����������Һ��������Һ�����ö����������������Ϊ��ɢ�ʿ�����С��ͬ |
| C����BaSO4Ͷ�뱥��Na2CO3��Һ��������BaCO3���ݴ˿�ȷ��Ksp��BaCO3��< Ksp��BaSO4 |
| D����NH4��2CO3��s��=NH4HCO3��s�� +NH3��g����H>0�����������Է�����Ϊ��ϵ��ֵ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
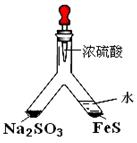
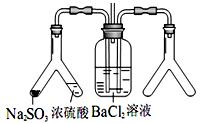
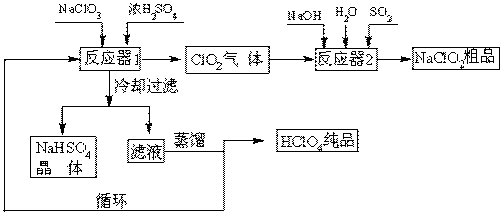
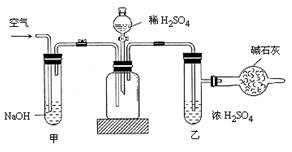
 ��Ŀ�ģ�̽�� SO2��BaCl2��Ӧ������������������������ͨ���Ȼ�����Һ����������������ͨ����һ���������Բ�����ɫ���������Ҳ� Y����Ӧ���õ�ҩƷ��____________��___________����Ҫʱ���Լ��ȣ����ó����Ļ�ѧʽΪ________________��
��Ŀ�ģ�̽�� SO2��BaCl2��Ӧ������������������������ͨ���Ȼ�����Һ����������������ͨ����һ���������Բ�����ɫ���������Ҳ� Y����Ӧ���õ�ҩƷ��____________��___________����Ҫʱ���Լ��ȣ����ó����Ļ�ѧʽΪ________________��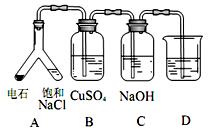
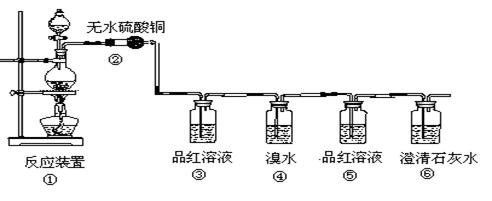
 ֤��ʯ������Ҫ�ɷ֣�D��ʢ��______________��
֤��ʯ������Ҫ�ɷ֣�D��ʢ��______________��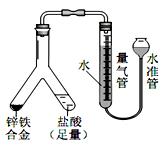
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A���ڸ������������ʯ�ң�����Ϊmg |
| B��ȡng��Ʒװ����ƿ�� |
| C�����װ�������� |
| D���ر�ֹˮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ��C
��C H
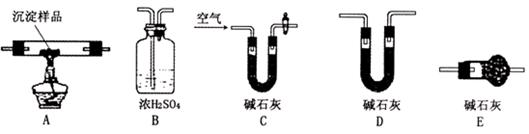
H �ı�����ϵ�����ʵ������ͼ��ʾ��
�ı�����ϵ�����ʵ������ͼ��ʾ��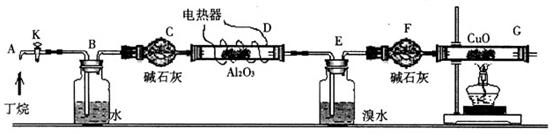
 D��Gװ�ü��ȣ��ڼ������װ�õ������ԣ����ų�װ���еĿ����ȡ���
D��Gװ�ü��ȣ��ڼ������װ�õ������ԣ����ų�װ���еĿ����ȡ���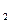 ��H
��H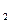 O��A
O��A l
l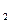 O
O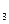 �������ѽ�Ĵ�����G����װ����ʡ�ԡ�
�������ѽ�Ĵ�����G����װ����ʡ�ԡ� ��C
��C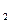 H
H

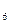 �����ʵ���֮��n(CH
�����ʵ���֮��n(CH )��n(C
)��n(C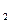 H
H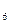 )��______________��
)��______________��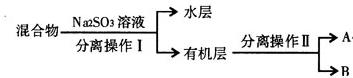

 SO
SO ��Һ��������______________________��
��Һ��������______________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| | ʵ�鲽�� | ʵ������ |
| �� | ����ĥ������Ƭ������������һ��Ũ�ȵ�CuCl2��Һ�С���ַ�Ӧ���ˡ� | �������ݣ��������ɵĺ�ɫ���壬��Һ��Ϊ��ɫ�� |
| �� | ��Һ�м���������NaOH��Һ�� | �а�ɫ���������� |
| �� | �����ú�ɫ����������ˮϴ�Ӻ��Ⱥ�ɡ� | ������ɫ������ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com