
 ��
������ ��1�����ݵ���ʽ����д��������д��
��2������������ͨʽ�ͷ�������ȷ���л����ʵķ���ʽ������д���������Ľṹ��ʽ��
��3�������Ժͽ����Ʒ�Ӧ�����Dz����������Ʒ�Ӧ��
��4������ԭ���غ�����ʽ��ȷ�����ش�
��5���˴Ź�������ֻ��2���壬���л������к����������͵ĵ�Ч��ԭ�ӣ�
��� �⣺��1���ǻ�����ԭ�Ӻ���ԭ��֮��ͨ�����ۼ��γɵ�9������������ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��2����Է�������Ϊ72�����������飬֧��Խ����е�Խ�ͣ����Էе���͵������ǣ�C��CH3��4��
�ʴ�Ϊ��C��CH3��4��
��3�������廯����C7H8O�����DZ��״�������������ʣ����У������Ժͽ����Ʒ�Ӧ�����Dz����������Ʒ�Ӧ��
�ʴ�Ϊ��C6H5-CH2OH��
��4���л���ȼ��ʱ���ĵ����������ɵ�CO2��H2O֮�����ʵ���֮��Ϊ1��2��2���������ʽΪCH2O2�����һ����HCOOH��
�ʴ�Ϊ��HCOOH��
��5����Է�������Ϊ58��ij��Ϊ���飬��˴Ź�������ֻ��2���壬�����������͵ĵ�Ч��ԭ�ӣ�
�ʴ�Ϊ��CH��CH3��3��CH3CH2CH2CH3��
���� ���⿼�����л���ṹ�����ʣ���Ŀ�Ѷ��еȣ���ȷ�����л���ṹ������Ϊ���ؼ���ע�����յ���ʽ������ʽ���ṹ��ʽ����дԭ������������ѧ�������Ӧ��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������




 ��W2Z
��W2Z ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
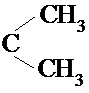 ��CH3CH2CH�TO+O�T
��CH3CH2CH�TO+O�T
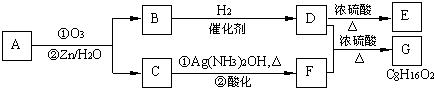
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ʵ���Ũ��֮��Ϊc��M����c��N����c��P��=2��2��3 | |
| B�� | ƽ����������Ƿ�Ӧ��ʼǰ��$\frac{3}{5}$ | |
| C�� | ƽ�������и����ʵ���Ũ����� | |
| D�� | ��λʱ���ڣ��������� a mol M ���ʣ���ͬʱҲ������ 1.5a mol P ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��طŵ�ʱ��Mg2+��Ǩ�� | |
| B�� | ��طŵ�ʱ��������ӦΪMo3S4+2xe-+xMg2+�TMgxMo3S4 | |
| C�� | Mg��������Mo3S4������ | |
| D�� | ������1mol Mo3S4����ת�Ƶĵ�����Ϊ2NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��й©�����ǿ�����Ժ�ǿ��ʴ�� | |
| B�� | ��Ԫ��λ�ڵ������ڢ�A�� | |
| C�� | ������С�մ���Һ��й©�����г�ϴ | |
| D�� | ʵ���ұ�������ʿ��Խ������ھƾ����л��ܼ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �۲��Ƿ���塢������������Ϊ��ˮ������������ΪӲˮ | |
| B�� | Ʒ���Ƿ�����ζ������ζ��Ϊ��ˮ������ζ��ΪӲˮ | |
| C�� | �÷���ˮ�������֣�������ĭ��Ϊ��ˮ����������ĭ��ΪӲˮ | |
| D�� | �ü��ȵķ����������֣�������ˮ����������ˮ����Ϊ��ˮ��������ˮ����ΪӲˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
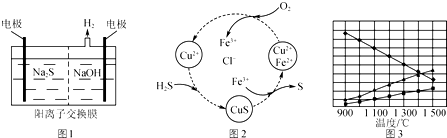
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | Fe2��SO4��3 | B�� | Ca��HCO3��2 | C�� | NH4HCO3 | D�� | ���ͳ���ʯ��ˮ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com