
 $��_{��}^{ŨH_{2}SO_{4}}$
$��_{��}^{ŨH_{2}SO_{4}}$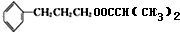 +H2O��
+H2O�� ��
��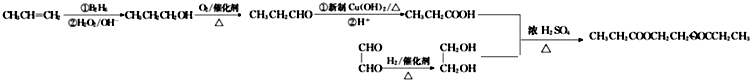 ��
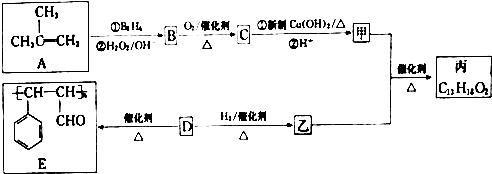
�� ���� ����A�Ľṹ��ʽ��֪��A����������Ϣ�еķ�Ӧ����BΪ��CH3��2CH2OH��B����������Ӧ����CΪ��CH3��2CHCHO��C�������ü�Ϊ��CH3��2CHCOOH������E�Ľṹ��ʽ��֪��D�����Ӿ۷�Ӧ����E������DΪ ��D�����������ӳɷ�Ӧ������Ϊ
��D�����������ӳɷ�Ӧ������Ϊ �������ҷ���������Ӧ���ɱ�Ϊ
�������ҷ���������Ӧ���ɱ�Ϊ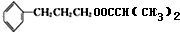 ���ݴ˴��⣮
���ݴ˴��⣮
��� �⣺����A�Ľṹ��ʽ��֪��A����������Ϣ�еķ�Ӧ����BΪ��CH3��2CH2OH��B����������Ӧ����CΪ��CH3��2CHCHO��C�������ü�Ϊ��CH3��2CHCOOH������E�Ľṹ��ʽ��֪��D�����Ӿ۷�Ӧ����E������DΪ ��D�����������ӳɷ�Ӧ������Ϊ
��D�����������ӳɷ�Ӧ������Ϊ �������ҷ���������Ӧ���ɱ�Ϊ
�������ҷ���������Ӧ���ɱ�Ϊ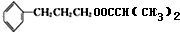 ��
��
��1������A�Ľṹ��ʽ��֪��A������Ϊ2-��-1-��ϩ��DΪ ��D�к���̼̼˫��������D��̼̼˫���ķ�����ȡ������D���Թ��У�����������������Һ��������Cu��OH��2����Һ�������ȣ�ȡ��Ӧ�����Һ����һ�Թ��У��������ữ���ټ���ˮ������ˮ��ɫ��֤������̼̼˫����
��D�к���̼̼˫��������D��̼̼˫���ķ�����ȡ������D���Թ��У�����������������Һ��������Cu��OH��2����Һ�������ȣ�ȡ��Ӧ�����Һ����һ�Թ��У��������ữ���ټ���ˮ������ˮ��ɫ��֤������̼̼˫����
�ʴ�Ϊ��2-��-1-��ϩ��ȡ������D���Թ��У�����������������Һ��������Cu��OH��2����Һ�������ȣ�ȡ��Ӧ�����Һ����һ�Թ��У��������ữ���ټ���ˮ������ˮ��ɫ��֤������̼̼˫����
��2��B��C�ķ�Ӧ������������Ӧ��D��E�ķ�Ӧ�����ǼӾ۷�Ӧ��
�ʴ�Ϊ��������Ӧ���Ӿ۷�Ӧ��
��3��CΪ��CH3��2CHCHO��C������Cu��OH��2����Һ��Ӧ�Ļ�ѧ����ʽΪ��CH3��2CHCHO+2Cu��OH��2+NaOH$\stackrel{��}{��}$��CH3��2CHCOONa+Cu2O��+3H2O��
�ʴ�Ϊ����CH3��2CHCHO+2Cu��OH��2+NaOH$\stackrel{��}{��}$��CH3��2CHCOONa+Cu2O��+3H2O��
��4�������ҷ�Ӧ���ɱ��Ļ�ѧ����ʽΪ ��CH3��2CHCOOH+ $��_{��}^{ŨH_{2}SO_{4}}$
$��_{��}^{ŨH_{2}SO_{4}}$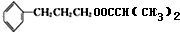 +H2O��
+H2O��
�ʴ�Ϊ����CH3��2CHCOOH+ $��_{��}^{ŨH_{2}SO_{4}}$
$��_{��}^{ŨH_{2}SO_{4}}$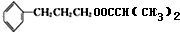 +H2O��
+H2O��
��5��DΪ ��������������������ͬ��ͬ���칹��Ϊ����������-C��CHO��=CH2��������-CHO��-CH=CH2�����ڼ�����֣����Թ���4 �֣�����һ��������DZ���һԪȡ����ں˴Ź���������������ҷ�����֮��Ϊ1��2��2��1��2����ͬ���칹��Ľṹ��ʽ��
��������������������ͬ��ͬ���칹��Ϊ����������-C��CHO��=CH2��������-CHO��-CH=CH2�����ڼ�����֣����Թ���4 �֣�����һ��������DZ���һԪȡ����ں˴Ź���������������ҷ�����֮��Ϊ1��2��2��1��2����ͬ���칹��Ľṹ��ʽ�� ��
��
�ʴ�Ϊ��4�� ��
��
��6���ɱ�ϩ���Ҷ�ȩΪԭ���Ʊ��������Ҷ���������ϩ������Ϣ�еķ�Ӧ���ɱ��������������ñ��ᣬ���Ҷ�ȩ��ԭ���Ҷ������������Ҷ�������������Ӧ�ö������Ҷ�������Ӧ�ĺϳ�·��Ϊ ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼���л���Ľṹ�����ʣ�Ϊ��Ƶ���㣬������ѧ���ķ��������Ŀ��飬ע������л���Ĺ����ŵ����ʣ�Ϊ������Ĺؼ����Ѷ��еȣ�


 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����к����ǻ�����������ˮ����Һһ�������� | |
| B�� | ���ᡢ�������������������Ϊͬ���칹�� | |
| C�� | �ܷ���������Ӧ��һ����ȩ | |
| D�� | �Ҵ�����������Ӧ����̼������ʧȥ�ǻ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | KNO3 | B�� | NH4Cl | C�� | NaHCO3 | D�� | NaHSO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���������Ӽ���ʳ������彡�����к�������ʳ�� | |
| B�� | ���������У�ȼ����ʹ��ѧ��ת��Ϊ���ܵĹؼ� | |
| C�� | �ܽ��ܵ�����Ҫ�ɷ־۰��������л��߷��ӻ����� | |
| D�� | ԭ�Ӿ����Է�Ӧ����ɫ��ѧ����Ҫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ����ʽΪC16H14O4 | |
| B�� | 1mol������X��һ�����������ӳ�6molH2 | |
| C�� | �����������ֹ����ţ�����������ˮ�������Ż������� | |
| D�� | 1mol������X�������2molNaOH��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
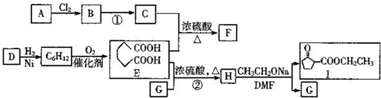
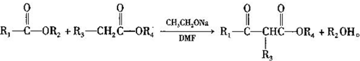
 ��1�й����ŵ������������ʻ���
��1�й����ŵ������������ʻ���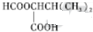 ��
�� д���ϳ�·��ͼ��
д���ϳ�·��ͼ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ��ѧ����ʽ | ��Ӧ���� |
| A | 4Fe��OH��2+O2+2H2O=4Fe��OH��3 | ���Ϸ�Ӧ |
| B | 2KMnO4$\frac{\underline{\;\;��\;\;}}{\;}$K2MnO4+MnO2+O2�� | �ֽⷴӦ |
| C | BaCl2+2AgNO3=2AgCl��+Ba��NO3��2 | ���ֽⷴӦ |
| D | Fe3O4+4CO$\frac{\underline{\;����\;}}{\;}$3Fe+4CO2 | �û���Ӧ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ����������ˮ | B�� | �ʵ������¶� | ||
| C�� | ��������CuSO4��Һ | D�� | ����������Ũ�Ƚϴ������ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com