
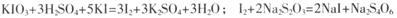
 Mn2����Cl2����2H2O ��2�֣�
Mn2����Cl2����2H2O ��2�֣�
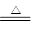 Mn2����Cl2����2H2O��
Mn2����Cl2����2H2O��

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������Ҵ������Ȼ�̼ | B���Ҵ�����ȩ�������� |
| C����ȩ�������������������� | D�����������Ҵ������� |
�鿴�𰸺ͽ���>>
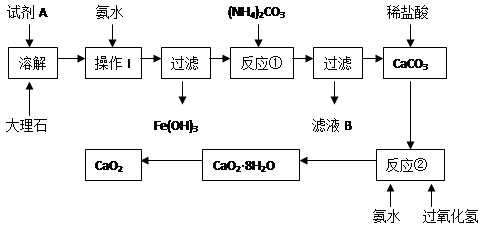
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
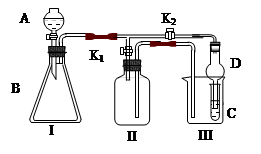
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ���� | ���� | ����������� | ������ͽ��۵� |
| �� | �ܽ� | ȡ20��NaNO3��17��KCl�ܽ���35mlˮ�У��������У������Ͻ��衣 | �����ܽ� |
| �� | ���� | �������Ƚ��裬ʹ��Һ����Ũ���� | �� �� ���������� |
| �� | �ȹ��� | ����Һ������ٵ�Լԭ����һ��ʱ��Ѹ�ٳ��ȹ��� | ��Һ�е�����Ҫ�ɷ�Ϊ �� �� |
| �� | ��ȴ | ����Һ��ȴ�����¡� | �о��������� |
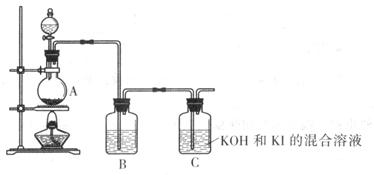
 �� �� | | ���й�Ҫ����в��� | �õ�����Ʒ����ؾ��� |
| �� | | ���õ��ij���Ʒ����ؾ�������������ˮ�У����ȡ����裬��ȫ���ܽ��ֹͣ���ȣ�ʹ��Һ��ȴ�����º���ˡ� | �õ����Ƚϸߵ�����ؾ��� |
| �� | ���� | �ֱ�ȡ�ݡ��õ��IJ�Ʒ�����ó���Һ��ֱ����1��1mol/l��HNO3��2��0.1mol/l��AgNO3 | �ɹ۲쵽�ݡ���Ʒ�г��ֵ�����ֱ��Ǣݲ�Ʒ�в�����ɫ��������Ʒ������������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ܢ� | B���ۢܢ� | C���ۢ� | D��ȫ�� |
�鿴�𰸺ͽ���>>
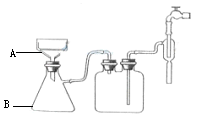
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Һ������㣬ʯ�����Ϊ��ɫ���л������ɫ |
| B��ʯ���Ϊ���㻷�����϶����������ϡ���ɫ |
| C��ʯ��������㣬�ϲ�Ϊ��ɫ���²�Ϊ��ɫ |
| D��ʯ���Ϊ���㻷�����϶����Ǻ졢�ϡ���ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����п��ϡ���ᷴӦ | B����п��Ũ���ᷴӦ |
| C����п��ϡ���ᷴӦ | D����п(��ͭ����)��ϡ���ᷴӦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Zn + 2HNO3(ϡ)= Zn(NO3)2 + H2�� |
B��CaCO3 + H2SO4(ϡ)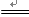 CaSO4 + H2O + CO2�� CaSO4 + H2O + CO2�� |
C��NH4Cl(aq) + NaOH(aq)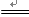 NaCl + H2O + NH3�� NaCl + H2O + NH3�� |
D��MnO2 + 4HCl(Ũ) 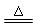 MnCl2 + Cl2��+ 2H2O MnCl2 + Cl2��+ 2H2O |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com