
 ����H ����������̼ԭ�Ӿ���һ��ֱ���ϣ���G ת��ΪH �Ļ�ѧ����ʽΪCH3CHBrCHBrCH3+2NaOH$��_{��}^{��}$H3CC��CCH3��+2NaBr+2H2O��
����H ����������̼ԭ�Ӿ���һ��ֱ���ϣ���G ת��ΪH �Ļ�ѧ����ʽΪCH3CHBrCHBrCH3+2NaOH$��_{��}^{��}$H3CC��CCH3��+2NaBr+2H2O�� ��
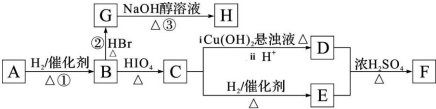
������ ����A������C��H��O����Ԫ�ص�������Ϊ6��1��4��֪C��H��O�����ʵ���֮��Ϊ2��4��1��������A������ͼ���ʺɱ����ֵΪ88������A�ķ���ʽΪC4H8O2�����ݷ���ʽ��֪A����һ�������Ͷȣ��ֲ���ʹBr2��CCl4��Һ��ɫ������A�д���C=O�����ٸ���1mol B��Ӧ������2mol C�������֪��������֪A�ĽṹΪ��CH3COCH��OH��CH3���������Խ�һ���Ƴ�B��C��D��E��F�Ľṹ��ʽ�����������ǣ�CH3CH��OH��CH��OH��CH3��CH3CHO��CH3COOH��CH3CH2OH��CH3COOCH2CH3������B��HBr����ȡ����Ӧ����±�������NaOH����Һ�з�����ȥ��Ӧ����H����������̼ԭ�Ӿ���һ��ֱ���ϣ����Կ����Ƶ�G��H�Ľṹ�ֱ�Ϊ��CH3CHBrCHBrCH3��H3CC��CCH3���ݴ˽��
��� �⣺����A������C��H��O����Ԫ�ص�������Ϊ6��1��4��֪C��H��O�����ʵ���֮��Ϊ2��4��1��������A������ͼ���ʺɱ����ֵΪ88������A�ķ���ʽΪC4H8O2�����ݷ���ʽ��֪A����һ�������Ͷȣ��ֲ���ʹBr2��CCl4��Һ��ɫ������A�д���C=O�����ٸ���1mol B��Ӧ������2mol C�������֪��������֪A�ĽṹΪ��CH3COCH��OH��CH3���������Խ�һ���Ƴ�B��C��D��E��F�Ľṹ��ʽ�����������ǣ�CH3CH��OH��CH��OH��CH3��CH3CHO��CH3COOH��CH3CH2OH��CH3COOCH2CH3������B��HBr����ȡ����Ӧ����±�������NaOH����Һ�з�����ȥ��Ӧ����H����������̼ԭ�Ӿ���һ��ֱ���ϣ����Կ����Ƶ�G��H�Ľṹ�ֱ�Ϊ��CH3CHBrCHBrCH3��H3CC��CCH3��
��1��������ķ�����֪��AΪCH3COCH��OH��CH3���ʴ�Ϊ��CH3COCH��OH��CH3��
��2��A�ṹ��ʽΪCH3COCH��OH��CH3����������������ʻ��ʹ��ǻ���
�ʴ�Ϊ���ʻ��ʹ��ǻ���
��3��D+E��F��ӦΪ�������Ҵ���Ӧ����������������Ӧ����Ϊ������Ӧ���ʴ�Ϊ��������Ӧ��
��4��������ķ�����֪��CΪ��ȩ������������Һ��Ӧ�����ӷ���ʽΪ��CH3CHO+2[Ag��NH3��2]++2OH- $\stackrel{��}{��}$CH3COO-+NH4++2Ag��+3NH3+H2O��
�ʴ�Ϊ��CH3CHO+2[Ag��NH3��2]++2OH- $\stackrel{��}{��}$CH3COO-+NH4++2Ag��+3NH3+H2O��
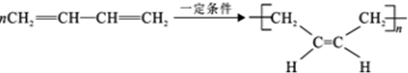
��5����H����������̼ԭ�Ӳ���һ��ֱ���ϣ���HΪ1��3-����ϩ��H��һ�������ºϳ�˳���Ļ�ѧ����ʽΪ ������B��2��3-����������HBr����ȡ����Ӧ����±����
������B��2��3-����������HBr����ȡ����Ӧ����±����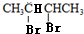 ������NaOH����Һ�з�����ȥ��Ӧ����H����H����������̼ԭ�Ӿ���һ��ֱ���ϣ����Կ����Ƶ�G��H�Ľṹ��GΪ
������NaOH����Һ�з�����ȥ��Ӧ����H����H����������̼ԭ�Ӿ���һ��ֱ���ϣ����Կ����Ƶ�G��H�Ľṹ��GΪ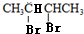 HΪ
HΪ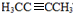 ��Gת��ΪH�Ļ�ѧ����ʽΪCH3CHBrCHBrCH3+2NaOH$��_{��}^{��}$H3CC��CCH3��+2NaBr+2H2O��
��Gת��ΪH�Ļ�ѧ����ʽΪCH3CHBrCHBrCH3+2NaOH$��_{��}^{��}$H3CC��CCH3��+2NaBr+2H2O��
�ʴ�Ϊ�� ��CH3CHBrCHBrCH3+2NaOH$��_{��}^{��}$H3CC��CCH3��+2NaBr+2H2O��
��CH3CHBrCHBrCH3+2NaOH$��_{��}^{��}$H3CC��CCH3��+2NaBr+2H2O��
��6��A�ĽṹΪ��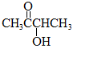 ������������
������������
a��X�˴Ź���������3���壬�����֮��Ϊ1��1��2����X������λ�õ��⣬�Ҹ���֮��Ϊ1��1��2��
b��1mol X����HIO4���ȵ������·�Ӧ�����γ�1mol ��Ԫȩ��������Ϣ��֪XӦΪ���������ǻ��Ļ�״�
c��1mol X�������2mol Na��Ӧ��˵��X��2���ǻ���d��X����NaHCO3��Ӧ��Ҳ����NaOH��Ӧ��Ҳ����Br2�����ӳɷ�Ӧ��˵����Xû���Ȼ���������̼̼˫������X�ĽṹΪ ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼���л����ƶϣ����ؿ���ѧ�������ƶ�������֪ʶǨ�����������ݷ�Ӧ�����������Ϣ��������˼ά�����ƶϣ��ѵ���ͬ���칹��ṹ��ʽ�жϣ���Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
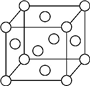 A��B��X��Y��Z��Ԫ�����ڱ�ǰ�������еij���Ԫ�أ�ԭ��������������AԪ�ؿ��γ���Ȼ��Ӳ�����ĵ��ʣ�B��Aͬ���ڣ�����������δ�ɶԵ��ӣ�Xԭ�ӵĵ�һ�����������ĵ����ֱܷ��ǣ�I1=578kJ/mol��I2=1 817kJ/mol��I3=2 745kJ/mol��I4=11 575kJ/mol�����³�ѹ�£�Y�����ǹ��壬�����������γ��������Ҫ���ʣ�Z��һ��ͬλ�ص�������Ϊ63��������Ϊ34����ش��������⣺
A��B��X��Y��Z��Ԫ�����ڱ�ǰ�������еij���Ԫ�أ�ԭ��������������AԪ�ؿ��γ���Ȼ��Ӳ�����ĵ��ʣ�B��Aͬ���ڣ�����������δ�ɶԵ��ӣ�Xԭ�ӵĵ�һ�����������ĵ����ֱܷ��ǣ�I1=578kJ/mol��I2=1 817kJ/mol��I3=2 745kJ/mol��I4=11 575kJ/mol�����³�ѹ�£�Y�����ǹ��壬�����������γ��������Ҫ���ʣ�Z��һ��ͬλ�ص�������Ϊ63��������Ϊ34����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
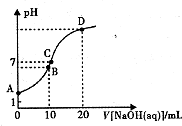 �����£���0.1 mol/LNaOH��Һ�ζ�10mL 0.1mol/LH2A��Һ����Һ��pH��NaOH��Һ�������ϵ��ͼ��ʾ������˵������ȷ���ǣ�������
�����£���0.1 mol/LNaOH��Һ�ζ�10mL 0.1mol/LH2A��Һ����Һ��pH��NaOH��Һ�������ϵ��ͼ��ʾ������˵������ȷ���ǣ�������| A�� | A����Һ�м�������ˮ��$\frac{c��O{H}^{-}��}{c��{H}_{2}A��}$���� | |
| B�� | B�㣺c�� HA -����c��H+����c��A2һ����c��H2A�� | |
| C�� | C�㣺c��Na+��=c��HA-��+2c�� A2-�� | |
| D�� | ˮ���������c��OH-����B�㣾D�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ù����������ᷴӦ�Ʊ��轺��SiO32-+2H+�TH2SiO3�����壩 | |
| B�� | FeI2��Һ��ͨ������Cl2��2Fe2++Cl2�T2Fe3++2Cl- | |
| C�� | ����NaHSO4 ��Һ��Ba��OH��2 ��Һ��Ӧ��H++OH-+SO42-+Ba2+�TH2O+BaSO4�� | |
| D�� | FeCl3��Һ��ͨ��SO2����Һ��ɫ��ȥ��2Fe3++SO2+2H2O�T2Fe2++SO42-+4H+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 7�� | B�� | 6�� | C�� | 5�� | D�� | 4�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
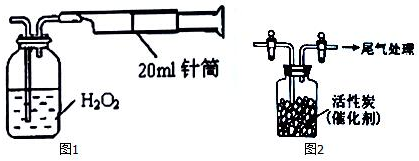
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com