
��25�桢101 kPa�£����ף���ѧʽΪP4�������ף���ѧʽΪP��ȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��P4(s) + 5O2(g)= P4O10(s)����H=��3093.2 kJ��mol-1
4P(s) + 5O2(g) = P4O10(s)����H =��2954.0 kJ��mol-1
�ɴ��ж�����˵����ȷ���ǣ� ��
A������ȼ����Ϊ2954.0 kJ��mol-1
B����֪������Ϊ��������ṹ����P-P��֮��ļн�Ϊ109��28��
C���ɺ���ת��Ϊ���������ȷ�Ӧ��������ʱ���������Ⱥ���
D���������İ��ͺ�������ͬ�����·ֱ�������������Ӧ�������ֻ��PCl5������ų�����������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
(20��)��Դ�ǹ��÷�չ����Ҫ�������ҹ�Ŀǰʹ�õ���Դ��Ҫ�ǻ�ʯȼ�ϡ�(1) ��25�桢101 kPaʱ��16 g CH4��ȫȼ������Һ̬ˮʱ�ų���������890.31 kJ����CH4ȼ�յ��Ȼ�ѧ����ʽΪ__________________________________________��
(2) ��֪��C(s)��O2(g)===CO2(g)����H����437.3 kJ��mol��1
H2(g)��O2(g)===H2O(g)����H����285.8kJ��mol��1
CO(g)��O2(g)===CO2(g)����H����283.0 kJ��mol��1
��ú������ӦC(s)��H2O(g)===CO(g)��H2(g) ���ʱ䦤H��_____________��
(3) ��¯������CO�������Ҫ��;֮һ���������ӦΪ��
FeO(s)��CO(g)Fe(s)��CO2(g) ��H>0����֪��1100��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=0.263��
�� �¶����ߣ���ѧƽ���ƶ���ﵽ�µ�ƽ�⣬��ʱƽ�ⳣ��Kֵ__________�����������С�����䡱����
�� 1100��ʱ��ø�¯�У�c(CO2)=0.025mol��L-1��c(CO)=0.1 mol��L-1��������������£��÷�Ӧ�Ƿ��ڻ�ѧƽ��״̬____________����ǡ��������ж�������______
____________________________________________________________��
(4) ����ͼ��ʾ��ɱպϻ�·�����У���װ����CH4Ϊ������O2��CO2�Ļ������Ϊ������ϡ����������Ϊ�缫��������̼����Ϊ����ʣ���װ����a��bΪʯī��b�����к�ɫ����������CuSO4��Һ�����Ϊ200 mL��
�� ��װ��������AΪ ���CH4����O2��CO2������d���ϵĵ缫��ӦʽΪ
_____________________________________��
�� ��װ����a���ϵĵ缫��ӦʽΪ__________________________________��
����a������112mL(��״��)���壬���װ��������CH4________ mL (��״��)����װ����������Һ��pH=__________�������Ե��ǰ����Һ����仯��
�� ������е缫���䣬����Һ���ɱ���Na2SO4��Һ������������a mol��������ʱ��ͬʱ��w g Na2SO4��10H2O�������������¶Ȳ��䣬ʣ����Һ�����ʵ���������ӦΪ__________________________(�ú�w��a�ı���ʽ��ʾ�����ػ���)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣�����̼ѭ�����Ѿ������˹�������ӣ��Իش��������⣺
��1��ú��������Һ���������ȼ�ϵ������ʡ�
��֪25�棬101![]() ʱ��
ʱ��![]()
![]()
����25�棬101![]() ʱ��
ʱ��![]() .
.
��2����¯������CO�������Ҫ��;֮һ���������ӦΪ��
����֪��1100��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=0.263��
���¶����ߣ���ѧƽ���ƶ���ﵽ�µ�ƽ�⣬��ʱƽ�ⳣ��Kֵ �����������С�����䡱����
��1100��ʱ��ø�¯ʱ��![]() ������������£��÷�Ӧ�Ƿ��ڻ�ѧƽ��״̬�� (��ǡ���)�����ж������� ��
������������£��÷�Ӧ�Ƿ��ڻ�ѧƽ��״̬�� (��ǡ���)�����ж������� ��
��Ŀǰ��ҵ�Ͽ���![]() ������ȼ�ϼ״����йط�ӦΪ��
������ȼ�ϼ״����йط�ӦΪ��
![]() ���������Ϊ1L���ܱ������У�����1mol
���������Ϊ1L���ܱ������У�����1mol![]() ��3mol
��3mol![]() ����Ӧ�����в��
����Ӧ�����в��![]() ��
��![]() (g)��Ũ����ʱ��ı仯��ͼ��ʾ��
(g)��Ũ����ʱ��ı仯��ͼ��ʾ��
�ٴӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ����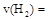 ��
��
�����д�ʩ��ʹ![]() ������� ������ţ���
������� ������ţ���
A.�����¶� B.�ٳ���![]() C.�ٳ���
C.�ٳ���![]()
D.��![]() ��g������ϵ�з��� E.����He(g),ʹ��ϵѹǿ����
��g������ϵ�з��� E.����He(g),ʹ��ϵѹǿ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡ����һ�и����ڶ���������⻯ѧ�Ծ� ���ͣ������
��10�֣�����̼ѭ�����Ѿ������˹�������ӣ��Իش��������⣺
��1��ú��������Һ���������ȼ�ϵ������ʡ�
��֪25�棬101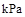 ʱ��
ʱ��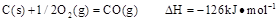
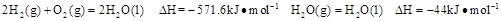
����25�棬101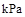 ʱ��
ʱ��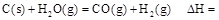 .
.
��2����¯������CO�������Ҫ��;֮һ���������ӦΪ�� ����֪��1100��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=0.263��
����֪��1100��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=0.263��
���¶����ߣ���ѧƽ���ƶ���ﵽ�µ� ƽ�⣬��ʱƽ�ⳣ��Kֵ �����������С�����䡱����
ƽ�⣬��ʱƽ�ⳣ��Kֵ �����������С�����䡱����
��1100��ʱ��ø�¯ʱ��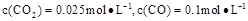 ������������£��÷�Ӧ�Ƿ��ڻ�ѧƽ��״̬�� (��ǡ���)�����ж������� ��
������������£��÷�Ӧ�Ƿ��ڻ�ѧƽ��״̬�� (��ǡ���)�����ж������� ��
��Ŀǰ��ҵ�Ͽ���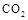 ������ȼ�ϼ״�
������ȼ�ϼ״� ���йط�ӦΪ��
���йط�ӦΪ��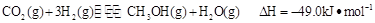 ���������Ϊ1L���ܱ������У�����1mol
���������Ϊ1L���ܱ������У�����1mol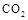 ��3mol
��3mol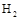 ����Ӧ�����в��
����Ӧ�����в��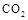 ��
�� (g)��Ũ����ʱ��ı仯��ͼ��ʾ��
(g)��Ũ����ʱ��ı仯��ͼ��ʾ��
�ٴӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ���� ��
��
�����д�ʩ��ʹ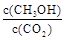 �������
�������  ������ţ���
������ţ���
| A�������¶� |
B���ٳ���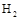 |
C���ٳ���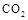 |
D����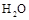 ��g������ϵ�з��� ��g������ϵ�з��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ������ѧ�ڼ���ѧϰЧ����⿼�Ի�ѧ�Ծ� ���ͣ������
(20��)��Դ�ǹ��÷�չ����Ҫ�������ҹ�Ŀǰʹ�õ���Դ��Ҫ�ǻ�ʯȼ�ϡ�(1) ��25�桢101 kPaʱ��16 g CH4��ȫȼ������Һ̬ˮʱ�ų���������890.31 kJ����CH4ȼ�յ��Ȼ�ѧ����ʽΪ__________________________________________��
(2) ��֪��C(s)��O2(g)===CO2(g)����H����437.3 kJ��mol��1
H2(g)��O2(g)===H2O(g)����H����285.8 kJ��mol��1
CO(g)��O2(g)===CO2(g)����H����283.0 kJ��mol��1
��ú������ӦC(s)��H2O(g)===CO(g)��H2(g) ���ʱ䦤H��_____________��
(3) ��¯������CO�������Ҫ��;֮һ���������ӦΪ��
FeO(s)��CO(g) Fe(s)��CO2(g) ��H>0����֪��1100��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=0.263��
Fe(s)��CO2(g) ��H>0����֪��1100��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=0.263��
�� �¶����ߣ���ѧƽ���ƶ���ﵽ�µ�ƽ�⣬��ʱƽ�ⳣ��Kֵ__________�����������С�����䡱����
�� 1100��ʱ��ø�¯�У�c(CO2)=0.025mol��L-1��c(CO)=0.1 mol��L-1��������������£��÷�Ӧ�Ƿ��ڻ�ѧƽ��״̬____________����ǡ��������ж�������______
____________________________________________________________��
(4) ����ͼ��ʾ��ɱպϻ�·�����У���װ����CH4Ϊ������O2��CO2�Ļ������Ϊ������ϡ����������Ϊ�缫��������̼����Ϊ����ʣ���װ����a��bΪʯī��b�����к�ɫ����������CuSO4��Һ�����Ϊ200 mL��
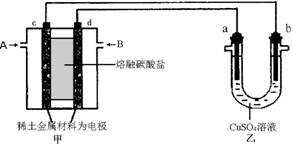
�� ��װ��������AΪ ���CH4����O2��CO2������d���ϵĵ缫��ӦʽΪ
_____________________________________��
�� ��װ����a���ϵĵ缫��ӦʽΪ__________________________________��
����a������112mL(��״��)���壬���װ��������CH4________ mL (��״��)����װ����������Һ��pH=__________�������Ե��ǰ����Һ����仯��
�� ������е缫���䣬����Һ���ɱ���Na2SO4��Һ������������a mol��������ʱ��ͬʱ��w g Na2SO4��10H2O�������������¶Ȳ��䣬ʣ����Һ�����ʵ���������ӦΪ__________________________(�ú�w��a�ı���ʽ��ʾ�����ػ���)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ�����ڶ���������⻯ѧ�Ծ� ���ͣ������
��10�֣�����̼ѭ�����Ѿ������˹�������ӣ��Իش��������⣺
��1��ú��������Һ���������ȼ�ϵ������ʡ�
��֪25�棬101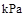 ʱ��
ʱ��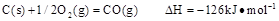
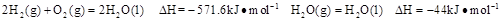
����25�棬101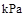 ʱ��
ʱ��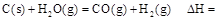 .
.
��2����¯������CO�������Ҫ��;֮һ���������ӦΪ��
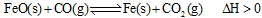 ����֪��1100��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=0.263��
����֪��1100��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=0.263��
���¶����ߣ���ѧƽ���ƶ���ﵽ�µ�ƽ�⣬��ʱƽ�ⳣ��Kֵ �����������С�����䡱����
��1100��ʱ��ø�¯ʱ��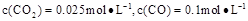 ������������£��÷�Ӧ�Ƿ��ڻ�ѧƽ��״̬�� (��ǡ���)�����ж�������
��
������������£��÷�Ӧ�Ƿ��ڻ�ѧƽ��״̬�� (��ǡ���)�����ж�������
��
��Ŀǰ��ҵ�Ͽ���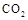 ������ȼ�ϼ״����йط�ӦΪ��
������ȼ�ϼ״����йط�ӦΪ��
 ���������Ϊ1L���ܱ������У�����1mol
���������Ϊ1L���ܱ������У�����1mol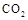 ��3mol
��3mol ����Ӧ�����в��
����Ӧ�����в��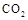 ��
��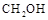 (g)��Ũ����ʱ��ı仯��ͼ��ʾ��
(g)��Ũ����ʱ��ı仯��ͼ��ʾ��
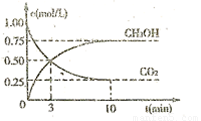
�ٴӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ���� ��
��
�����д�ʩ��ʹ ������� ������ţ���
������� ������ţ���
A.�����¶� B.�ٳ��� C.�ٳ���
C.�ٳ���
D.�� ��g������ϵ�з���
E.����He(g),ʹ��ϵѹǿ����
��g������ϵ�з���
E.����He(g),ʹ��ϵѹǿ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com