
| ʵ����� | ʵ������ | ������ͣ������ӷ���ʽ��ʾ�� | |
| ̽���� | ��3�� |
��ҺpH=8 | ��4�� |
| ̽���� | ��������ˮ��pH��2���еμ�����Na2S2O3��Һ | ��ˮ��ɫ��dz | ��5�� |


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
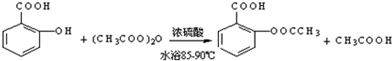
 ����������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128��135�森ijѧϰС����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[��CH3CO��2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣��Ʊ����������������£�
����������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128��135�森ijѧϰС����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[��CH3CO��2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣��Ʊ����������������£�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ�����и�����һ�������Ի�ѧ�Ծ� ���ͣ�ʵ����
��14�֣�ijѧϰС����ͨ����ӦNa2S2O3+H2SO4=Na2SO4+S��+SO2��+H2O�о���Ӧ���ʵ�Ӱ�����غ�Na2S2O3��������Ȥ��������֪Na2S2O3����Ϊ��������ƣ��׳ƺ��������Կ�������һ��Sԭ��ȡ����Na2SO4�е�һ��Oԭ�Ӷ��γɡ���ʵ��С���������ѧϰ��˼��Ԥ����Na2S2O3��ijЩ���ʣ���ͨ��ʵ��̽����֤�Լ���Ԥ�⡣
[�������]
��1������ѧ����ΪNa2S2O3��Na2SO4�ṹ���ƣ���ѧ����Ҳ���ƣ��������ʱNa2S2O3��Һ��pH 7���>������=����<����
��2������ѧ����SԪ�ػ��ϼ��Ʋ�Na2S2O3��SO2�������ƣ������н�ǿ�� ��
[ʵ��̽��]
ȡ����Na2S2O3���壬����ˮ���Ƴ�Na2S2O3��Һ����������̽������д�±��пո�
[ʵ�����]
��6��̽���٣� ��
��7��̽���ڣ� ��
[��������]
��8����ͬѧ��̽���ڡ���Ӧ�����Һ�еμ���������Һ���۲쵽�а�ɫ�������������ݴ���Ϊ��ˮ�ɽ�Na2S2O3����������Ϊ�÷����Ƿ���ȷ��˵������
��
��9�������������һ��ʵ�鷽����֤��Na2S2O3����ˮ��������ķ�����
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ�����и�����һ�������Ի�ѧ�Ծ� ���ͣ�ʵ����
��14�֣�ijѧϰС����ͨ����ӦNa2S2O3+H2SO4=Na2SO4+S��+SO2��+H2O�о���Ӧ���ʵ�Ӱ�����غ�Na2S2O3��������Ȥ��������֪Na2S2O3����Ϊ��������ƣ��׳ƺ��������Կ�������һ��Sԭ��ȡ����Na2SO4�е�һ��Oԭ�Ӷ��γɡ���ʵ��С���������ѧϰ��˼��Ԥ����Na2S2O3��ijЩ���ʣ���ͨ��ʵ��̽����֤�Լ���Ԥ�⡣
[�������]
��1������ѧ����ΪNa2S2O3��Na2SO4�ṹ���ƣ���ѧ����Ҳ���ƣ��������ʱNa2S2O3��Һ��pH 7���>������=����<����
��2������ѧ����SԪ�ػ��ϼ��Ʋ�Na2S2O3��SO2�������ƣ������н�ǿ�� ��
[ʵ��̽��]
ȡ����Na2S2O3���壬����ˮ���Ƴ�Na2S2O3��Һ����������̽������д�±��пո�

[ʵ�����]
��6��̽���٣� ��
��7��̽���ڣ� ��
[��������]
��8����ͬѧ��̽���ڡ���Ӧ�����Һ�еμ���������Һ���۲쵽�а�ɫ�������������ݴ���Ϊ��ˮ�ɽ�Na2S2O3����������Ϊ�÷����Ƿ���ȷ��˵������
��
��9�������������һ��ʵ�鷽����֤��Na2S2O3����ˮ��������ķ�����
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣�ijѧϰС����ͨ����ӦNa2S2O3+H2SO4=Na2SO4+S��+SO2��+H2O�о���Ӧ���ʵ�Ӱ�����غ�Na2S2O3��������Ȥ��������֪Na2S2O3����Ϊ��������ƣ��׳ƺ��������Կ�������һ��Sԭ��ȡ����Na2SO4�е�һ��Oԭ�Ӷ��γɡ���ʵ��С���������ѧϰ��˼��Ԥ����Na2S2O3��ijЩ���ʣ���ͨ��ʵ��̽����֤�Լ���Ԥ�⡣
[�������]
��1������ѧ����ΪNa2S2O3��Na2SO4�ṹ���ƣ���ѧ����Ҳ���ƣ��������ʱNa2S2O3��Һ��pH 7���>������=����<����
��2������ѧ����SԪ�ػ��ϼ��Ʋ�Na2S2O3��SO2�������ƣ������н�ǿ�� ��
[ʵ��̽��]
ȡ����Na2S2O3���壬����ˮ���Ƴ�Na2S2O3��Һ����������̽������д�±��пո�
[ʵ�����]
��6��̽���٣� ��
��7��̽���ڣ� ��
[��������]
��8����ͬѧ��̽���ڡ���Ӧ�����Һ�еμ���������Һ���۲쵽�а�ɫ�������������ݴ���Ϊ��ˮ�ɽ�Na2S2O3����������Ϊ�÷����Ƿ���ȷ��˵������
��
��9�������������һ��ʵ�鷽����֤��Na2S2O3����ˮ��������ķ�����
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com