
����Ŀ��һѧ�����������ʵ�鷽������NaCl��CaCl2���ֹ�������
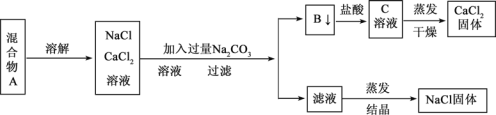
�ش���������
��1�������з�Ӧ�����ӷ���ʽ�� __________��________________��
��2������ʵ�鷽������õ���NaCl���������������ʣ�����Ϊ����������ijһ�����ë������һ������ȷ��Ʒ���Ӧ��____________________________��
��3����Ҫ�ⶨ����Ʒ��NaCl��CaCl2�������ȣ��ɳ��������B ���ʺ���һ���ʵ�����������������__________________���������ʶ�Ӧ����ĸ��
��4��ʵ������м���ϴ�� B �����Ƿ�ϴ���ķ�����___________________��
��5����ʵ���������Ҫ0.1mol/L��Na2CO3��Һ450 mL��ʵ����ʦ��̼���ƾ���(Na2CO3��10H2O)���Ƹ�Ũ�ȵ���Һ������Э����ʦ�����������:
�� ����Na2CO3��Һʱ��Ҫ����Ҫ�����������ձ�����������___________________��
�� ��ʵ��ĵ�һ���Ǽ��㣬��ȡ_________g̼���ƾ��塣
�� ������ʱ���ӿ̶��ߣ��������Ƶ���ҺŨ��_____________���ƫ�ߡ���ƫ�͡����䡱����
���𰸡�Ca2+ + CO32- = CaCO3�� CaCO3 + 2H+ = Ca2+ + CO2��+ H2O ������Һ�м����������������ٲ���������������ᾧ A ȡ���һ��ϴ��Һ�������еμ�ϡ�������������Һ�����ް�ɫ������������B������ϴ�� ��ͷ�ιܡ�500 mL ����ƿ 14.3 g ƫ��
��������
��1���������漰������Ӧ��һ����CaCl2��Na2CO3��Ӧ����һ����CaCO3�����ᷴӦ��
��2����Ϊ���ڳ���Ca2+��Na2CO3������������Һ�б�Ȼ����Na2CO3���ɼ���ȥ����
��3����Ϊ�ڷ�Ӧ������������NaCl��������Ҫ�ⶨ����Ʒ��NaCl��CaCl2�������ȣ���������ԭ��Ʒ��������
��4������ϴ�� B �����Ƿ�ϴ���ķ����Ǽ���ϴ��Һ���Ƿ������Һ�е�ij�����ӡ�
��5����Ϊʵ����û��450 mL������ƿ������ѡ��500mL������ƿ��
�� ����Na2CO3��Һʱȱ�ٵ���������ʵ��������з�����
�� ����500mL������ƿ������ʱ��Һ�������Ӧ��500mL��
�� ������ʱ���ӿ̶��ߣ�����������Һ�����ƫ��Ũ�ȱ仯�����ù�ʽ���з�����
��1���������漰������Ӧ��һ����CaCl2��Na2CO3��Ӧ�����ӷ���ʽΪCa2+ + CO32- = CaCO3������һ����CaCO3�����ᷴӦ�����ӷ���ʽΪCaCO3 + 2H+ = Ca2+ + CO2��+ H2O����Ϊ��Ca2+ + CO32- = CaCO3����CaCO3 + 2H+ = Ca2+ + CO2��+ H2O��
��2����Ϊ���ڳ���Ca2+��Na2CO3������������Һ�б�Ȼ����Na2CO3���ɼ���ȥ������ȷ�IJ���Ϊ��������Һ�м����������������ٲ���������������ᾧ����Ϊ��������Һ�м����������������ٲ���������������ᾧ��
��3����Ϊ�ڷ�Ӧ������������NaCl��������Ҫ�ⶨ����Ʒ��NaCl��CaCl2�������ȣ���������ԭ��Ʒ����������Ϊ��A��
��4��ֻ�ܼ���ϴ��Һ���Ƿ����Cl-������Ϊȡ���һ��ϴ��Һ�������еμ�ϡ�������������Һ�����ް�ɫ������������B������ϴ������Ϊ��ȡ���һ��ϴ��Һ�������еμ�ϡ�������������Һ�����ް�ɫ������������B������ϴ����
��5����Ϊʵ����û��450 mL������ƿ������ѡ��500mL������ƿ��
�� ����Na2CO3��Һʱ��ȱ�ٵ�����Ϊ��ͷ�ιܡ�500 mL ����ƿ����Ϊ����ͷ�ιܡ�500 mL ����ƿ��
��m(Na2CO3��10H2O)= 0.1mol/L��0.5L��286g/mol=14.3g������14.3 g��
�� ������ʱ���ӿ̶��ߣ�����������Һ�����ƫ�ߣ���c=![]() ��֪��Vƫ�ߣ���cƫ�͡���Ϊ��ƫ�͡�
��֪��Vƫ�ߣ���cƫ�͡���Ϊ��ƫ�͡�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA���������ӵ�����������˵������ȷ���ǣ� ��
A.18gˮ�к��е���ԭ����ĿΪNA
B.1 mol�������������ԭ����ĿΪ2NA
C.106g̼�����к��е�������Ϊ2NA
D.��0.5 mol HNO3��������Һ�к��е���ԭ��Ϊ1.5 NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(NH4)2SO4�dz����Ļ��ʺͻ���ԭ�ϣ������ֽ⡣ij��ȤС����̽����ֽ���
[��������](NH4)2SO4��260���400��ʱ�ֽ���ﲻͬ��
[ʵ��̽��]��С����ѡ����ͼ��ʾװ�ý���ʵ�飨�гֺͼ���װ���ԣ�
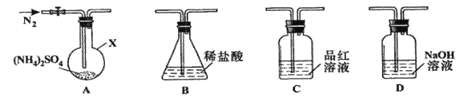
ʵ��1������װ��A-B-C-D����������ԣ���ͼʾ�����Լ���װ��Bʢ0.5000mol/L����70.00mL����ͨ��N2�ž���������260�����װ��Aһ��ʱ�䣬ֹͣ���ȣ���ȴ��ֹͣͨ��N2��Ʒ����Һ����ɫ��ȡ��װ��B������ָʾ������0.2000mol/L NaOH��Һ�ζ�ʣ�����ᣬ�յ�ʱ����NaOH��Һ25.00 mL��������ζ������Һ����SO42-��
��1������X��������________________��
��2���ζ�ǰ�����в�������ȷ˳����_________(����ĸ���)��
a��ʢװ0.2000mol/L NaOH��Һ
b����0.2000mol/L NaOH��Һ��ϴ
c����������¼
d����©����ϴ
e���ž��ζ��ܼ�������ݲ�����Һ��
��3��װ��B����Һ������������ʵ�����__________mol��
ʵ��2������װ��A-D-B����������ԣ���ͼʾ���¼����Լ���ͨ��N2�ž���������400�����װ��A��(NH4)2SO4��ȫ�ֽ������ֹͣ���ȣ���ȴ��ֹͣͨ��N2���۲쵽װ��A��D֮��ĵ���������������ɫ���塣�����飬�ð�ɫ�����װ��D����Һ����SO32-����SO42-����һ���о����֣�����������������
��4������װ��D����Һ����SO32-����SO42-��ʵ�������������__________��
��5��װ��B����Һ���յ�������____________��
��6��(NH4)2SO4��400��ֽ�Ļ�ѧ����ʽ��______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼΪһ������ȼ�ϵ�ؽṹʾ��ͼ�����ڸõ��������ȷ����

A. �������ΪCm(H2O)n������һ��������
B. �������ڵ缫���ŵ�ʱ����������Ӧ
C. �ŵ�����У�H����������������
D. ������ӦʽΪMnO2��4H����2e��=Mn2����2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��V mL Al2(SO4)3��Һ�к���Al3+ a g��ȡ0.5V mL��Һϡ�͵�8V mL����ϡ�ͺ���Һ��SO42�������ʵ���Ũ����
A. ![]() mol/L B.
mol/L B. ![]() mol/L C.
mol/L C. ![]() mol/L D.
mol/L D. ![]() mol/L
mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����״���µļ����һ����̼�Ļ������8.96 L����������7.60 g�����������м�������Ϊ________��һ����̼������Ϊ_______��
��2������CO��CO2�Ļ�����壬�������������ش�����
�����û�������ڱ�״���µ��ܶ�Ϊ1.79 g/L������������CO���������Ϊ__________��
������״���£�2.24 L�û�����������Ϊ4 g������������CO��CO2�����ʵ���֮��Ϊ_________��
��3��һ�����ļ�����O2����������ȼ�գ��õ�CO��CO2��H2O����������7.2 g��������ˮ������Ϊ3.6 g����CO��������_________��
��4����һ�������£�ij���廯����X���ȷֽⷽ��ʽΪ: 2X = A��+2B��+3C������÷�Ӧ�����ɵĻ�������H2������ܶ�Ϊ11������ͬ�����£�X����Է���������_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ���ǣ�������
A. ʯ�ͷ������������Ʊ�����ϩ����ȡ���¶ȼƵ�λ�ö�����ͬ
B. �����ʵ������Һ�м�ˮ���ɹ۲쵽��Һ������Ϊ�б��㡢ˮ����屽��
C. ±������±ԭ�ӵļ��飬������±����ˮ���IJ�����ֱ�ӵμ�![]() ��Һ
��Һ
D. �Ҵ���Ũ���ᷴӦ���Ƶõ�����ͨ�뵽��ˮ�У�����ˮ��ɫ����֤������ϩ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������MnO2��10mol/L������100mL����������Ӧ��ȡCl2������Ӧ��������0.2mol���ӷ���ת�ƣ���
��1�����ɱ�״����Cl2������Ƕ��٣�
��2����������HClռԭ��HCl�����ʵ����ٷ����Ƕ��٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1�����������ܵ������___�����ڵ���ʵ���___��
��NaCl���� ��Һ̬SO2 ��Һ̬���� ��ͭ ��BaSO4���� ������(C12H22O11) �߰�ˮ ���ۻ���KNO3
��2��0.5molCH4��������___g���ڱ�״���µ����Ϊ___L��
��3��8.4g������9.6gij����Rx����ԭ�Ӹ�����ͬ���ҷ��Ӹ���֮��Ϊ3��2����x��ֵ��___��R��Ħ��������___��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com