
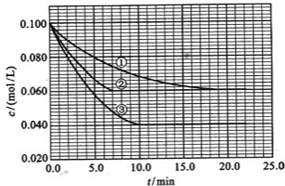
����Һ�У���ӦA+2B![]() C�ֱ������ֲ�ͬʵ�������½��У����ǵ���ʼŨ�Ⱦ�Ϊ
C�ֱ������ֲ�ͬʵ�������½��У����ǵ���ʼŨ�Ⱦ�Ϊ![]() ��
��![]() ��
��![]() ����Ӧ��A��Ũ����ʱ��ı仯����ͼ��ʾ��
����Ӧ��A��Ũ����ʱ��ı仯����ͼ��ʾ��
��ش��������⣺
��1����ٱȽϣ��ں͢۷ֱ���ı�һ�ַ�Ӧ���������ı���������жϵ������ǣ�
��____________ ��
��_______________ ��
��2��ʵ���ƽ��ʱB��ת����Ϊ________��ʵ���ƽ��ʱC��Ũ��Ϊ__________��
��3���÷�Ӧ��![]() _______0���ж���������________________________________��
_______0�����������________________________________��
��4���÷�Ӧ���е�4.0minʱ��ƽ����Ӧ���ʣ�
ʵ��ڣ�![]() =_______________________��ʵ��ۣ�
=_______________________��ʵ��ۣ�![]() =____________________��
=____________________��
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
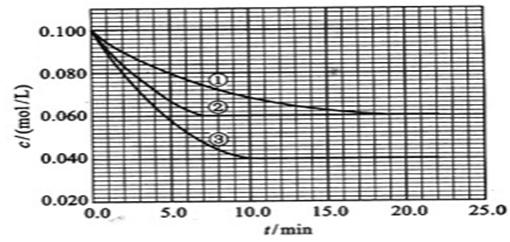
 ��2010?����������Һ�У���ӦA+2B?C�ֱ������ֲ�ͬʵ�������½��У����ǵ���ʼŨ�Ⱦ�Ϊc��A��=0.100mol/L��c��B��=0.200mol/L �� c��C��=0mol/L����Ӧ��A��Ũ����ʱ��ı仯��ͼ��ʾ��
��2010?����������Һ�У���ӦA+2B?C�ֱ������ֲ�ͬʵ�������½��У����ǵ���ʼŨ�Ⱦ�Ϊc��A��=0.100mol/L��c��B��=0.200mol/L �� c��C��=0mol/L����Ӧ��A��Ũ����ʱ��ı仯��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
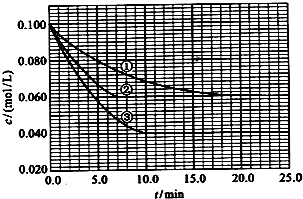
 ����Һ�У���ӦA+2B?C�ֱ������ֲ�ͬʵ�������½��У����ǵ���ʼŨ�Ⱦ�Ϊ c��A��=1.0mol/L��c��B��=2.0mol/L��c��C��=0mol/L����Ӧ��A��Ũ����ʱ��ı仯��ͼ��ʾ����ش��������⣺
����Һ�У���ӦA+2B?C�ֱ������ֲ�ͬʵ�������½��У����ǵ���ʼŨ�Ⱦ�Ϊ c��A��=1.0mol/L��c��B��=2.0mol/L��c��C��=0mol/L����Ӧ��A��Ũ����ʱ��ı仯��ͼ��ʾ����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����Һ�У���ӦA+2B?C�ֱ������ֲ�ͬʵ�������½��У����ǵ���ʼŨ�Ⱦ�Ϊc��A��=0.100mol/L��c��B��=0.200mol/L��c��C��=0mol/L����Ӧ��A��Ũ����ʱ��ı仯��ͼ��ʾ��
����Һ�У���ӦA+2B?C�ֱ������ֲ�ͬʵ�������½��У����ǵ���ʼŨ�Ⱦ�Ϊc��A��=0.100mol/L��c��B��=0.200mol/L��c��C��=0mol/L����Ӧ��A��Ũ����ʱ��ı仯��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��������һ����Ҫ�����ȼ�ϣ�Ҳ�������������������ȣ��Գ��������ƻ����ã���ҵ�Ͽ�����ú���������ˮú�����ϳɶ����ѣ�
��������һ����Ҫ�����ȼ�ϣ�Ҳ�������������������ȣ��Գ��������ƻ����ã���ҵ�Ͽ�����ú���������ˮú�����ϳɶ����ѣ�| ���� | CH3OH | CH3OCH3 | H2O |
| Ũ��/��mol?L-1�� | 0.44 | 0.6 | 0.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
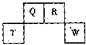 ��2012?������һģ��������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ���ش��������⣺
��2012?������һģ��������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ���ش��������⣺

| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com