
��16�֣���1��0.02mol/L��CH3COOH��Һ��0.02mol/L CH3COONa��Һ�������ϣ���֪�û����Һ�У�c(H+)>c(OH-)���á�>��<��=��������գ�
����Һ��c(Na+)_____c(CH3COO-) �� c(CH3COO-)_______ c(CH3COOH)
�� c(CH3COOH)+c(CH3COO-) 0.04 mol/L
��2����HnA���B(OH)m��ȫ��Ӧ��������.
����HnAΪHCl���Ҹ�����Һ��pH��7�������ӷ���ʽ˵��ԭ��
������0.4mol��L��1��NaOH��Һ��0.2mol��L��1��HnA��Һ�������Ϻ�pH=10��
��HnAΪ ������ţ�.
a.һԪǿ�� b. һԪ���� c. ��Ԫǿ�� d. ��Ԫ����
��3��ijѧУ������ȤС��Ӻ�ˮɹ�κ����±(��Ҫ��Na+��Mg2+��Cl����Br����)��ģ�ҵ��������ȡþ����Ҫ�������£��ش��������⣺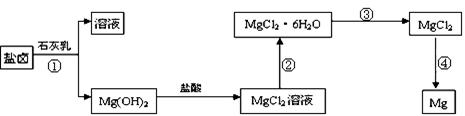
��.�ӹ��̢ٵõ���Mg(OH)2�����л���������Ca(OH)2 ����ȥ����Ca(OH)2�ķ������Ƚ��������뵽ʢ�� ��Һ���ձ��У���ֽ�����ˡ�ϴ�ӿɵô�����Mg(OH)2��
��.���̢۵�ת����Ҫ��HCl�����м��ȣ�HCl��������
��.д�����̢��з�����Ӧ�Ļ�ѧ����ʽ
 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��16�֣���1��0.02mol/L��CH3COOH��Һ��0.02mol/LCH3COONa��Һ�������ϣ���֪�û����Һ�У�c(H+)>c(OH-)���á�>��<��=��������գ�
����Һ��c(Na+)_____c(CH3COO-)�� c(CH3COO-)_______ c(CH3COOH)
�� c(CH3COOH)+c(CH3COO-) 0.04 mol/L
��2����HnA���B(OH)m��ȫ��Ӧ��������.
����HnAΪHCl���Ҹ�����Һ��pH��7�������ӷ���ʽ˵��ԭ��
������0.4mol��L��1��NaOH��Һ��0.2mol��L��1��HnA��Һ�������Ϻ�pH=10��
��HnAΪ ������ţ�.
a.һԪǿ�� b. һԪ���� c. ��Ԫǿ�� d. ��Ԫ����
��3��ijѧУ������ȤС��Ӻ�ˮɹ�κ����±(��Ҫ��Na+��Mg2+��Cl����Br����)��ģ�ҵ��������ȡþ����Ҫ�������£��ش��������⣺
��.�ӹ��̢ٵõ���Mg(OH)2�����л���������Ca(OH)2����ȥ����Ca(OH)2�ķ������Ƚ��������뵽ʢ�� ��Һ���ձ��У���ֽ�����ˡ�ϴ�ӿɵô�����Mg(OH)2��
��.���̢۵�ת����Ҫ��HCl�����м��ȣ�HCl��������
��.д�����̢��з�����Ӧ�Ļ�ѧ����ʽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ӱ�ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
���淴Ӧ N2��g��+3H2��g�� 2NH3��g������500��ʱ����2molN2 ��2molH2�����ݻ�Ϊ10L���ܱ������н��з�Ӧ���ﵽƽ��ʱ��NH3�����ܴﵽ��Ũ���ǣ� ��
2NH3��g������500��ʱ����2molN2 ��2molH2�����ݻ�Ϊ10L���ܱ������н��з�Ӧ���ﵽƽ��ʱ��NH3�����ܴﵽ��Ũ���ǣ� ��
A. 0.01mol��L��1 B. 0.02mol��L��1
C. 0.05mol��L��1 D. 0.15mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�츣��ʡ����Ϫһ�и߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��16�֣���1��0.02mol/L��CH3COOH��Һ��0.02mol/L CH3COONa��Һ�������ϣ���֪�û����Һ�У�c(H+)>c(OH-)���á�>��<��=��������գ�
����Һ��c(Na+)_____c(CH3COO-) �� c(CH3COO-)_______ c(CH3COOH)
�� c(CH3COOH)+c(CH3COO-) 0.04 mol/L
��2����HnA���B(OH)m��ȫ��Ӧ��������.
����HnAΪHCl���Ҹ�����Һ��pH��7�������ӷ���ʽ˵��ԭ��
������0.4mol��L��1��NaOH��Һ��0.2mol��L��1��HnA��Һ�������Ϻ�pH=10��
��HnAΪ ������ţ�.
a.һԪǿ�� b. һԪ���� c. ��Ԫǿ�� d. ��Ԫ����
��3��ijѧУ������ȤС��Ӻ�ˮɹ�κ����±(��Ҫ��Na+��Mg2+��Cl����Br����)��ģ�ҵ��������ȡþ����Ҫ�������£��ش��������⣺
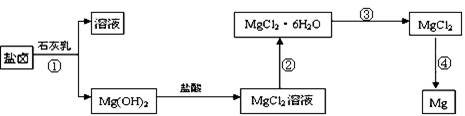
��.�ӹ��̢ٵõ���Mg(OH)2�����л���������Ca(OH)2 ����ȥ����Ca(OH)2�ķ������Ƚ��������뵽ʢ�� ��Һ���ձ��У���ֽ�����ˡ�ϴ�ӿɵô�����Mg(OH)2��
��.���̢۵�ת����Ҫ��HCl�����м��ȣ�HCl��������
��.д�����̢��з�����Ӧ�Ļ�ѧ����ʽ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com