
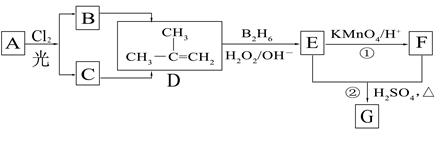
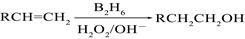 £ØB2H6ĪŖŅŅÅšĶ飩
£ØB2H6ĪŖŅŅÅšĶ飩 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
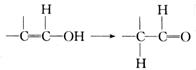

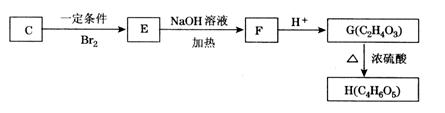
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
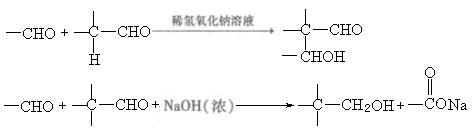
 £Br£©æÉŅŌŗĶ½šŹō·“Ӧɜ³ÉĢž»ł½šŹōÓŠ»ś»ÆŗĻĪļ”£ŗóÕßÓÖÄÜÓėŗ¬ōŹ»ł»ÆŗĻĪļ·“Ӧɜ³É“¼£ŗ
£Br£©æÉŅŌŗĶ½šŹō·“Ӧɜ³ÉĢž»ł½šŹōÓŠ»ś»ÆŗĻĪļ”£ŗóÕßÓÖÄÜÓėŗ¬ōŹ»ł»ÆŗĻĪļ·“Ӧɜ³É“¼£ŗ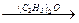 RMgBr
RMgBr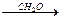 RCH2OMgBr
RCH2OMgBr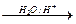 RCH2OH
RCH2OH
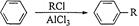
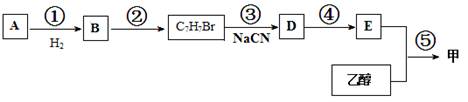
 »ł±¾µÄŹÆÓĶ²śĘ·£ØŅŅĻ©”¢±ūĻ©”¢±ūĶ锢±½µČ£©ŗĶ
»ł±¾µÄŹÆÓĶ²śĘ·£ØŅŅĻ©”¢±ūĻ©”¢±ūĶ锢±½µČ£©ŗĶ ČĪŃ”ĪŽ»śŹŌ¼ĮĪŖŌĮĻŅĄĻĀĮŠĀ·ĻßŗĻ³ÉB£¬ŗĻ³ÉBµÄĀ·ĻßĪŖ£ŗ
ČĪŃ”ĪŽ»śŹŌ¼ĮĪŖŌĮĻŅĄĻĀĮŠĀ·ĻßŗĻ³ÉB£¬ŗĻ³ÉBµÄĀ·ĻßĪŖ£ŗ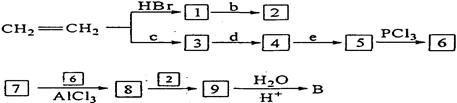
 £ŗ______________£»
£ŗ______________£»²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
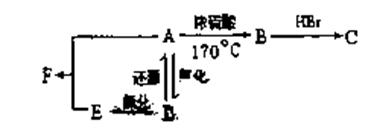
 £Ø2£©»ÆŗĻĪļCµÄ½į¹¹¼ņŹ½ĪŖ
£Ø2£©»ÆŗĻĪļCµÄ½į¹¹¼ņŹ½ĪŖ ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
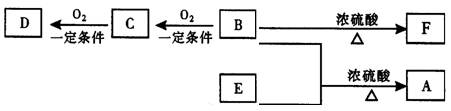
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
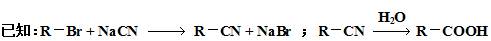
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗ¼ĘĖćĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
 £¬”ŖRµČ£¬æÉŹ¹ŠĀµ¼ČėµÄČ”“ś»ł½ųČė±½»·µÄĮŚĪ»ŗĶ¶ŌĪ»£»µŚ¶žĄąČē”ŖNO2”¢”ŖSO3H”¢”ŖCHOµČ£¬æÉŹ¹ŠĀµ¼ČėµÄČ”“ś»ł½ųČė±½»·µÄ¼äĪ»”£µ±µŚŅ»ĄąŗĶµŚ¶žĄąĶ¬Ź±“ęŌŚŹ±£¬ŅŌµŚŅ»ĄąĪŖ×¼”£
£¬”ŖRµČ£¬æÉŹ¹ŠĀµ¼ČėµÄČ”“ś»ł½ųČė±½»·µÄĮŚĪ»ŗĶ¶ŌĪ»£»µŚ¶žĄąČē”ŖNO2”¢”ŖSO3H”¢”ŖCHOµČ£¬æÉŹ¹ŠĀµ¼ČėµÄČ”“ś»ł½ųČė±½»·µÄ¼äĪ»”£µ±µŚŅ»ĄąŗĶµŚ¶žĄąĶ¬Ź±“ęŌŚŹ±£¬ŅŌµŚŅ»ĄąĪŖ×¼”£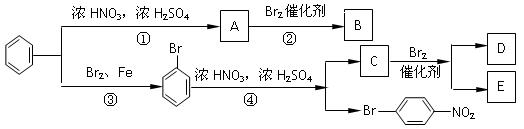
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com