
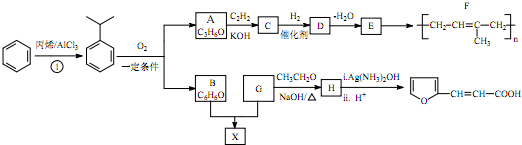
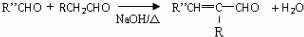 ��-R��-R�䡢-R���ʾ������ͬ����ܲ�ͬ��ԭ�ӻ�ԭ���ţ�
��-R��-R�䡢-R���ʾ������ͬ����ܲ�ͬ��ԭ�ӻ�ԭ���ţ� ��
�� ��H��ͬ���칹�������ڷ����廯����Ĺ���5�֣�
��H��ͬ���칹�������ڷ����廯����Ĺ���5�֣�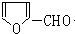 +CH3CHO$��_{��}^{NaOH}$
+CH3CHO$��_{��}^{NaOH}$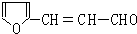 +H2O��
+H2O��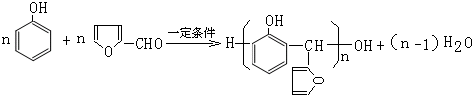 ��
�� ���� �ڴ��������£���ϩ�ͱ������ӳɷ�Ӧ���� ��
�� ��Ӧ����B��A��B��FeCl3��Һ��������ɫ������B�ķ���ʽ��֪��BΪ���ӣ�A����Ȳ�ܷ�����Ӧ����D��A�����к˴Ź����������շ�ֻ��1�飬����A�ķ���ʽ֪��A��CH3COCH3��CH3COCH3����Ȳ�����ӳɷ�Ӧ����C��C�Ľṹ��ʽΪ��
��Ӧ����B��A��B��FeCl3��Һ��������ɫ������B�ķ���ʽ��֪��BΪ���ӣ�A����Ȳ�ܷ�����Ӧ����D��A�����к˴Ź����������շ�ֻ��1�飬����A�ķ���ʽ֪��A��CH3COCH3��CH3COCH3����Ȳ�����ӳɷ�Ӧ����C��C�Ľṹ��ʽΪ�� ��C�����������ӳɷ�Ӧ����D��D������ȥ��Ӧ����E�����F�Ľṹ��ʽ֪��E�Ľṹ��ʽΪ��CH2=CHC��CH3��=CH2��D�Ľṹ��ʽΪ��
��C�����������ӳɷ�Ӧ����D��D������ȥ��Ӧ����E�����F�Ľṹ��ʽ֪��E�Ľṹ��ʽΪ��CH2=CHC��CH3��=CH2��D�Ľṹ��ʽΪ��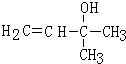 ��G����ȩ��Ӧ����H��H��������Һ��ӦȻ���ữ����
��G����ȩ��Ӧ����H��H��������Һ��ӦȻ���ữ����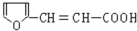 ������H�Ľṹ��ʽΪ��
������H�Ľṹ��ʽΪ��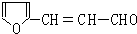 �����������Ϣ֪��G�Ľṹ��ʽΪ��
�����������Ϣ֪��G�Ľṹ��ʽΪ��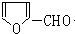 ��G�ͱ��ӷ�Ӧ����X����X�Ľṹ��ʽΪ��
��G�ͱ��ӷ�Ӧ����X����X�Ľṹ��ʽΪ��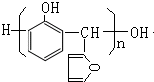 �������л���Ľṹ�����ʽ��
�������л���Ľṹ�����ʽ��
��� �⣺�ڴ��������£���ϩ�ͱ������ӳɷ�Ӧ���� ��
�� ��Ӧ����B��A��B��FeCl3��Һ��������ɫ������B�ķ���ʽ��֪��BΪ���ӣ�A����Ȳ�ܷ�����Ӧ����D��A�����к˴Ź����������շ�ֻ��1�飬����A�ķ���ʽ֪��A��CH3COCH3��CH3COCH3����Ȳ�����ӳɷ�Ӧ����C��C�Ľṹ��ʽΪ��
��Ӧ����B��A��B��FeCl3��Һ��������ɫ������B�ķ���ʽ��֪��BΪ���ӣ�A����Ȳ�ܷ�����Ӧ����D��A�����к˴Ź����������շ�ֻ��1�飬����A�ķ���ʽ֪��A��CH3COCH3��CH3COCH3����Ȳ�����ӳɷ�Ӧ����C��C�Ľṹ��ʽΪ�� ��C�����������ӳɷ�Ӧ����D��D������ȥ��Ӧ����E�����F�Ľṹ��ʽ֪��E�Ľṹ��ʽΪ��CH2=CHC��CH3��=CH2��D�Ľṹ��ʽΪ��
��C�����������ӳɷ�Ӧ����D��D������ȥ��Ӧ����E�����F�Ľṹ��ʽ֪��E�Ľṹ��ʽΪ��CH2=CHC��CH3��=CH2��D�Ľṹ��ʽΪ��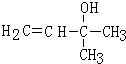 ��G����ȩ��Ӧ����H��H��������Һ��ӦȻ���ữ����
��G����ȩ��Ӧ����H��H��������Һ��ӦȻ���ữ����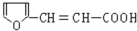 ������H�Ľṹ��ʽΪ��
������H�Ľṹ��ʽΪ��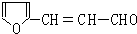 �����������Ϣ֪��G�Ľṹ��ʽΪ��
�����������Ϣ֪��G�Ľṹ��ʽΪ��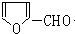 ��G�ͱ��ӷ�Ӧ����X����X�Ľṹ��ʽΪ��
��G�ͱ��ӷ�Ӧ����X����X�Ľṹ��ʽΪ�� ��
��
��1����������ķ�����֪���ٷ�Ӧ������Ϊ�ӳɷ�Ӧ��
�ʴ�Ϊ���ӳɷ�Ӧ��
��2����������ķ�����֪��A��CH3COCH3��
�ʴ�Ϊ��CH3COCH3��
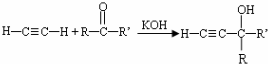
��3��A��CΪCH3COCH3����Ȳ�����ӳɷ�Ӧ����C����Ӧ�Ļ�ѧ����ʽCH3COCH3+CH��CH$\stackrel{KOH}{��}$ ��
��
�ʴ�Ϊ��CH3COCH3+CH��CH$\stackrel{KOH}{��}$ ��
��
��4��D�Ľṹ��ʽΪ��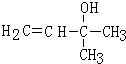 ��
��
a��D����ʽΪC5H10O����a����
b��D���ǻ�̼��û���⣬���ɱ�������ȩ�࣬��b����
c��D�й����ź���̼̼˫�����ǻ�����c��ȷ��
d��D����̼̼˫�����ǻ����ܹ������ӳɡ���ȥ��ȡ�����ۺϵȷ�Ӧ����d��ȷ��
��ѡ��ab��
��5��H�Ľṹ��ʽΪ��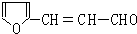 ���䷴ʽ�ṹ��ʽΪ
���䷴ʽ�ṹ��ʽΪ  ��H��ͬ���칹�������ڷ����廯����ĽṹΪ����������-OH��-CHO�����ڼ�����֣�������-OOCH����-COOH������5�֣�
��H��ͬ���칹�������ڷ����廯����ĽṹΪ����������-OH��-CHO�����ڼ�����֣�������-OOCH����-COOH������5�֣�
�ʴ�Ϊ�� ��5��
��5��
��6��G�Ľṹ��ʽΪ��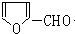 ��G��H�Ļ�ѧ����ʽΪ
��G��H�Ļ�ѧ����ʽΪ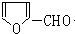 +CH3CHO$��_{��}^{NaOH}$
+CH3CHO$��_{��}^{NaOH}$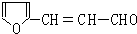 +H2O��
+H2O��
�ʴ�Ϊ��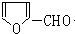 +CH3CHO$��_{��}^{NaOH}$
+CH3CHO$��_{��}^{NaOH}$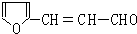 +H2O��
+H2O��
��7��BΪ���ӣ�B�� G��һ�������·�Ӧ����X�Ļ�ѧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼���л�����ƶϣ�ע���������Ϣ�������ơ��������ϵķ������з�����ע������ŵı仯���ѵ���ͬ���칹��������жϣ��ѶȽϴ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���

 ��
��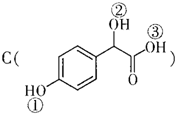 �Т١��ڡ���3��-OH��������ǿ������˳���ǣ��ۣ��٣��ڣ�
�Т١��ڡ���3��-OH��������ǿ������˳���ǣ��ۣ��٣��ڣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
 ��1��Ŀǰ��ҵ�ϳɰ���ԭ����N2��g��+3H2��g��?2NH3��g����H=-93.0kJ•mol-1��
��1��Ŀǰ��ҵ�ϳɰ���ԭ����N2��g��+3H2��g��?2NH3��g����H=-93.0kJ•mol-1���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��CH3��3CCH2OH | B�� | 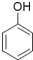 | C�� |  | D�� | ��CH3��2CBrCH2OH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ѧ�仯һ��������µĺ��� | |
| B�� | ������ˮ�����漰��ѧ�仯 | |
| C�� | �����ܸı仯ѧ��Ӧ�Ļ�ܺ��ʱ� | |
| D�� | ��ʵ����������м������ͭ��ϡ����Ϊԭ���Ʊ�ͭ������������;���� ;��a��Fe$\stackrel{H_{2}SO_{4}}{��}$H2$��_{��}^{Cu}$Cu����;��b��CuO$\stackrel{H_{2}SO_{4}}{��}$CuSO4$\stackrel{Fe}{��}$Cu ʵ�ʲ����У��Ƶõ�������ͭʱ��;��a��;��b����Fe������һ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ӵ����ģ�ͣ�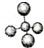 | B�� | NH4Br�ĵ���ʽ��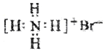 | ||
| C�� | ��ԭ�ӵĽṹʾ��ͼ�� | D�� | ���ǻ�������Ľṹ��ʽ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Ϊ�л��� | B�� | ������Ϊ���� | ||
| C�� | ������������Ԫ����� | D�� | ������ȼ�ղ�����ܺ�CO2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com