
 2BO3����һ���̶��ݻ�Ϊ2L���ܱ������г���0.20mol��BO2��0.10mol��O2������Ӻ�ﵽƽ�⣬��������к�BO3Ϊ0.18mol����v��o2 ��=______mol?L-1?min-1��������ͨ��0.20mol BO2��0.10mol O2���ٴδﵽ��ƽ���BO3�����ʵ�������______֮�䣮
2BO3����һ���̶��ݻ�Ϊ2L���ܱ������г���0.20mol��BO2��0.10mol��O2������Ӻ�ﵽƽ�⣬��������к�BO3Ϊ0.18mol����v��o2 ��=______mol?L-1?min-1��������ͨ��0.20mol BO2��0.10mol O2���ٴδﵽ��ƽ���BO3�����ʵ�������______֮�䣮 Al��OH��3+H2S+KCl����֪A��B��C��D����Ԫ��ΪAl��S��Cl��K��A��B��C��D����Ԫ��ԭ�ӵĺ˵������������AΪAl��BΪS��CΪCl��DΪK����A+C��E��֪��EΪAlCl3����B+D��F��֪��FΪK2S��
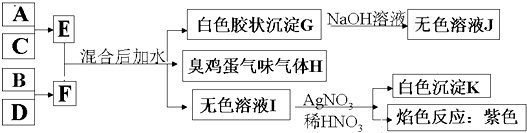
Al��OH��3+H2S+KCl����֪A��B��C��D����Ԫ��ΪAl��S��Cl��K��A��B��C��D����Ԫ��ԭ�ӵĺ˵������������AΪAl��BΪS��CΪCl��DΪK����A+C��E��֪��EΪAlCl3����B+D��F��֪��FΪK2S��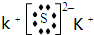 ��
��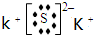 ��
�� Al2O3+2H2O�����յõ��Ĺ���ΪAl2O3��
Al2O3+2H2O�����յõ��Ĺ���ΪAl2O3��  Al2O3+2H2O��
Al2O3+2H2O�� =0.18mol/��L?min��������֮�ȵ��ڻ�ѧ������֮�ȣ���v��O2 ��=
=0.18mol/��L?min��������֮�ȵ��ڻ�ѧ������֮�ȣ���v��O2 ��= v��SO3��=
v��SO3��= ��0.18mol/��L?min��=0.09mol/��L?min����
��0.18mol/��L?min��=0.09mol/��L?min���� Al��OH��3+H2S+KCl����֪A��B��C��D����Ԫ��ΪAl��S��Cl��K��A��B��C��D����Ԫ��ԭ�ӵĺ˵������������AΪAl��BΪS��CΪCl��DΪK����A+C��E��֪��EΪAlCl3����B+D��F��֪��FΪK2S��
Al��OH��3+H2S+KCl����֪A��B��C��D����Ԫ��ΪAl��S��Cl��K��A��B��C��D����Ԫ��ԭ�ӵĺ˵������������AΪAl��BΪS��CΪCl��DΪK����A+C��E��֪��EΪAlCl3����B+D��F��֪��FΪK2S��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�




| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�013

A. 4x
B. 4x��6
C. 4x��10
D. 4x��14
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�013
ԭ������ΪJ��Ԫ�������ڱ���λ��A��B��C��D����Ԫ�ص��м䣬A��B��C��D����Ԫ���ǣ���ϵ���ϵ������Ԫ�س��⣩���� ��

A. 4x
B. 4x��6
C. 4x��10
D. 4x��14
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��D���ֶ�����Ԫ�أ�ԭ������D>A>B>C����A��Bͬ���ڣ�C��Dͬ���壬A��ԭ�ӽṹʾ��ͼΪ��  ��B��C���γ����ӻ�����
��B��C���γ����ӻ�����![]() ���ݴ���գ�
���ݴ���գ�
![]() (1)A��Ԫ������Ϊ ������̬������Ļ�ѧʽΪ ��
(1)A��Ԫ������Ϊ ������̬������Ļ�ѧʽΪ ��
![]() (2)A��B��C��D����Ԫ����ԭ�裬�뾶��С�����˳��Ϊ ��
(2)A��B��C��D����Ԫ����ԭ�裬�뾶��С�����˳��Ϊ ��
![]() (3)B��C����������ˮ���ﻯѧʽ�ֱ�Ϊ �� ��
(3)B��C����������ˮ���ﻯѧʽ�ֱ�Ϊ �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(6��)A��B��C��D���ֶ�����Ԫ�أ�ԭ������D>A>B>C����A��Bͬ���ڣ�C��Dͬ���壬A��ԭ�ӽṹʾ��ͼΪ��  ��B��C���γ����ӻ�����
��B��C���γ����ӻ�����![]() ���ݴ���գ�
���ݴ���գ�
(1)A��Ԫ������Ϊ ������̬������Ļ�ѧʽΪ ��
(2)A��B��C��D����Ԫ����ԭ�裬�뾶��С�����˳��Ϊ ��
(3)B��C����������ˮ���ﻯѧʽ�ֱ�Ϊ �� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com