
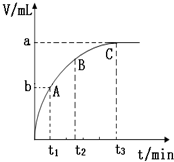
 ijͬѧ��ʵ���ҽ���ʵ���о���ѧ��Ӧ���ʵ�Ӱ�����أ�
ijͬѧ��ʵ���ҽ���ʵ���о���ѧ��Ӧ���ʵ�Ӱ�����أ�Ũ �� ��Ӧ���� | 30% H2O2 | 15% H2O2 | 10% H2O2 | 5% H2O2 |
| ������������ | ��������Ӧ | ��������Ӧ | ��������Ӧ | ��������Ӧ |
| �������� �� | 360 | 480 | 540 | 720 |
| MnO2���������� | 10 | 25 | 60 | 120 |
���� ��1�����������ڴ������������·ֽ�����ˮ��������
��2������������Һ��ʵ������ȡ��������ҪҩƷ���ڳ����º��ѷֽ�õ���������ֽ��ٶ���Ũ�ȡ��¶ȡ����������ص�Ӱ�����ʵ�鷽����֤��ʱ��Ҫע��ʵ��Ŀ��Ʊ�������ȷ��ʵ������ȷ�ԣ�
��3���ֱ�����¶ȡ�Ũ�ȡ������Է�Ӧ���ʵ�Ӱ��ǶȽ��
��4���������ʵı�ʾ������ͼ���������������������
��5����������MnO2�����ữ��H2O2����Һ�У�MnO2�ܽ����Mn2+��ͬʱ��������������ˮ���ݴ�д����Ӧ�����ӷ���ʽ��
�٢ٸ���ʵ��ҩƷ�IJ�ͬ�����Ӱ�����أ�����v=$\frac{��V}{��t}$ȷ����¼���ݣ�
�ڲ����¶ȵ��������¶ȼƣ�
��� �⣺��1����������ֽ�Ļ�ѧ����ʽΪ��2H2O2$\frac{\underline{\;MnO_{2}\;}}{\;}$2H2O+O2����
�ʴ�Ϊ��2H2O2$\frac{\underline{\;MnO_{2}\;}}{\;}$2H2O+O2����
��2�����ݱ��и��������ݣ����������ȵ�����£���ͬŨ�ȵĹ���������Һ���Ǽ�������Ӧ�����������ȵ�����£���ͬŨ�ȵĹ���������Һ���ֽ⣬˵����������ķֽ��������¶��йأ����ǵõ���ͬ�����ʱ�䲻ͬ��Ũ��Խ��Ӧ���ٶ�Խ�죬˵����������ķֽ�������Ũ���йأ��Ƚ�ͬһŨ�ȵĹ���������Һ��30%ʱ�����������ȵ�ʱ����Ҫʱ����360s���д������ȵ������£���Ҫʱ����10s��˵����������ķֽ��������¶ȡ������йأ�
�ʴ�Ϊ���¶ȣ�������
��3�������������ݿ��Կ�����Ũ��Խ��Ӧ����Խ�죬�����ܼӿ��������ķֽ⣬�д����Ƿֽ����ʿ죬
�ʴ�Ϊ���¶�����ѧ��Ӧ���ʼӿ죨��Ӧ��Ũ������ѧ��Ӧ���ʼӿ죻ʹ�ú��ʵĴ�����ѧ��Ӧ���ʼӿ죩��
��4����ͼ��������ʾʱ�䣬�������ʾ��������������ʱ��Խ�����ɵ�����Խ�࣬��Ӧ����Խ�죬��������������ΪC��
�ʴ�Ϊ��C��
��5����������MnO2�����ữ���H2O2��Һ�У�MnO2�ܽ����Mn2+��ͬʱ����������ˮ���÷�Ӧ�����ӷ���ʽΪ��MnO2+H2O2+2H+=Mn2++O2��+2H2O��
�ʴ�Ϊ��MnO2+2H++H2O2=Mn2++O2��+2H2O��
�ٸ����ṩ��ҩƷ֪�������Ũ�Ȳ�ͬ�����ı������ͬ�������о��Ļ�ѧ��Ӧ���ʵ�Ӱ�����������Ũ�Ⱥ����ı������
����v=$\frac{��V}{��t}$֪��Ϊ�˱��ڹ۲죬��¼������Ӧ���Ƿ�Ӧʱ�䣬
�ʴ�Ϊ�������Ũ�ȡ����ı��������Ӧ��ʼ��ֹͣ���õ�ʱ�䣻
����Ҫ�о��¶ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죬����Ҫ�����¶ȵ�����-�¶ȼƣ�
�ʴ�Ϊ���¶ȼƣ�
���� ���⿼��̽����������Է�Ӧ���ʵ�Ӱ�죬��Ŀ�Ѷ��еȣ�ע����ƶ���ʵ��ʱ����������������ͬ��ֻ��һ��������ͬʱ���ܵó���ȷ���ۣ����������¶ȡ�Ũ�ȡ������������Է�Ӧ���ʵ�Ӱ��Ϊ���ؼ���


 ���б�ˢ��ϵ�д�
���б�ˢ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �ü��顢�״����Ҵ������ѵȿ����Ƴ�ȼ�ϵ�أ�ʵ�����У����Լ״�ȼ�ϵ��Ϊֱ����Դ����һ��Ũ�ȵ���ȩ�� Na2SO4��ҺΪ�������Һ��ģ����ȩ��ˮ�Ĵ������̣���ԭ������ȩ�ֱ�����������������Ӧ��ת��Ϊ�Ҵ������ᣬ��װ��ʾ��ͼ��ͼ��ʾ��
�ü��顢�״����Ҵ������ѵȿ����Ƴ�ȼ�ϵ�أ�ʵ�����У����Լ״�ȼ�ϵ��Ϊֱ����Դ����һ��Ũ�ȵ���ȩ�� Na2SO4��ҺΪ�������Һ��ģ����ȩ��ˮ�Ĵ������̣���ԭ������ȩ�ֱ�����������������Ӧ��ת��Ϊ�Ҵ������ᣬ��װ��ʾ��ͼ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ʾ�ķ���ʽΪC6H14�ṹ��ʽ��CH3��2CHCH2CH2CH3������2-�����飮
��ʾ�ķ���ʽΪC6H14�ṹ��ʽ��CH3��2CHCH2CH2CH3������2-�����飮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���Ӷ������� | B�� | �����¶� | C�� | �����¶� | D�� | �Ƴ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | C6H10 | B�� | C4H8 | C�� | C4H10O | D�� | C5H11Cl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com