
�����Ƿ�����������FeS2���������ַ������������IJ�������ͼ���£�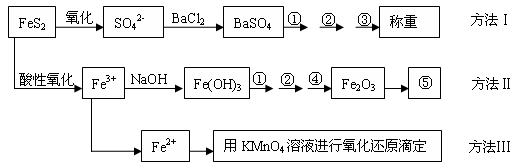
��ش��������⣺
(1)����ͼ�в����١��ڡ��۷ֱ�ָ���Ǣ�_________����__________����________��
�����ܡ����õ�����Ҫ�����ǣ���__________����__________(ÿ����1-2������)��
(2)�ж���Һ��SO42-�����ѳ�����ȫ�ķ�����_________________________________��
(3)ijͬѧ�÷�����ⶨ������FeԪ�صĺ�����ȷ��ȡһ�����Ŀ�ʯ�������������ܽ⡢Ԥ������
| A���ô��п̶ȵ��ձ����Ƴ�100 mL������Һ�� | B������Ͳ��ȡ25.00 mL������Һ�� | C����������ƿ�С� | D��������ˮϴ�ӵζ��ܺ�װ��KMnO4����Һ���øñ���Һ�ζ�����������(E)����Һ��ɵ��Ϻ�ɫʱ��ֹͣ�ζ�����30���ڲ���ɫ��(F)��ȡ������ζ��������ĵ�KMnO4����Һ��������������е�FeԪ�غ�������ָ����ʵ������д����������ı��________________________�� |
��1������ ��1�֣� ϴ�� ��1�֣� ���� ��1�֣� �������ƾ��� ��1�֣� ��ƽ ��1�֣�
��2��ȡ�ϲ���Һ�μ�BaCl2��Һ�����ް�ɫ�������ɣ�˵��SO42-������ȫ ��2�֣�
��3��A��B��D ��3�֣�
��4����2�֣����������ܵ�ԭ��д������һ�֣�����2�֣�
�� Fe(OH)3���������������������
�ڹ���ϴ��ʱδ��ֽ�����������ϴȥ
�� Fe(OH)3���ղ���֣�δ��ȫת��ΪFe2O3
��5��75.0% ��3�֣�
���������������1������Һ�еõ������Ĺ����辭�����ˡ�ϴ�ӡ������������ڢܲ�Ϊ����ʹ���������ֽ�Ϊ��������Ȼ�����������������ȷ��FeS2����������ʹ�õ������ֱ�Ϊ�������ƾ��ƺ���ƽ����2��ȡ�ϲ���Һ�μ�BaCl2��Һ�����ް�ɫ�������ɣ�˵��SO42-������ȫ����3��A��������ҺҪ������ƿ������B����Ͳ�Ķ���ֻ�ܾ�ȷ��ʮ��λ������D���ζ���Ҫ�ô�װҺ��ϴ������4�������Ĺ��������ߣ����½��ƫ�ߣ��ʿ��ܵ�ԭ���У��� Fe(OH)3��������������������� �ڹ���ϴ��ʱδ��ֽ�����������ϴȥ �� Fe(OH)3���ղ���֣�δ��ȫת��ΪFe2O3��
��5��n(FeS2)=n(SO42-)/2=2n(BaSO4)/2=4.66��233��2=0.01mol
�ÿ�ʯ��FeS2������������0.01��120��1.60=0.75
���㣺���鶨��ʵ��Ļ�����������������������й����⡣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��8�֣���NaCl��������1mol/L��NaCl��Һ100mL��
��1��ʵ�����������������ٲ��������ڽ�ͷ�ιܣ���100mL��Ͳ����100mL����ƿ����250mL��Ͳ����������ƽ����50mL�ձ���Ӧѡ�õ����������ţ� ��
��2��Ӧ��ȡNaCl������Ϊ ��
(3)�ڶ���ʱ����С�ĵμӵ�����ˮ�����˿̶��ߣ������ķ����� ����NaCl��Һת�Ƶ�����ƿ��δ���ձ��Ͳ���������ϴ�ӣ���������Һ��Ũ��(�ƫ�ߡ���ƫ�͡����䡱) ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijУ��ѧС���ͬѧ��չ��һϵ�еĻ�ѧʵ����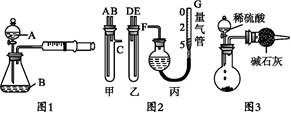
�������ʵ�鲢�����������:
(1)��ͬѧ��ͼ1��ʾװ��,����п�����ᷴӦ����:��2 gп��������ƿ��,ͨ����Һ©������1 mol��L-1ϡ����40 mL,�ռ�10 mL����,ͨ����¼�������������õ���Ӧ����Ϊx mol��(L��min)-1��ʵ�鿪ʼʱ����װ�������Եķ�������������������������������
(2)��ͬѧ��ͼ2װ�òⶨNa2CO3��NaCl�Ĺ���������Na2CO3����������:
�ټס������Թܸ�����������,�������Ӷ�Ӧ�ӿں�,����ʢϡ������Թ�,������Ӧ,�ų�����,����������ϡ����Ӧ�ֱ�����������������������(���������);
��G�ܿ����û�ѧʵ�������һ�ֳ������������,����������������;
�������ס��ҽӿڵ����ӷ�ʽ����:A��������,B����������,C����������(��д���ӿڵı��);
��Ϊ��߲�����ȷ��,�ռ��������,��װ�ö���ǰӦ���еIJ�����������������������������������������������������
(3)��ͬѧ���ͬѧʵ��Ŀ����ͬ:��ͼ3װ�òⶨ���ɵ�CO2������,����װ�ô�������ȱ��,�Ӷ�����ʵ�����,�����������ʹ�ⶨ�������ƫ�����Ҫԭ��������������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
Ϊ̽����ҵ���ϵ������ã�ij��ѧ��ȤС�������������ͼʵ�鷽�����ú�����������ͭ�ĺϽ���ȡ�Ȼ������̷�����(FeSO4��7H2O)�͵������塣 
��ش�
��1��������о�����е�ʵ������� ���ò����г��õ��ձ��Ͳ������⣬�������õ��IJ��������� ��
��2���Լ�X��
��3��������з�����Ӧ�����ӷ���ʽ�ǣ�_______________________________________
��4�����в����ʱ����С����������ͼ��ʾװ�ü��Լ����Ƶõ�CO2����ͨ����ҺA�С�һ��ʱ��۲쵽�ձ��в����İ�ɫ�������٣�Ϊ�˱������C���٣��Ľ��Ĵ�ʩ����װ�â�֮������һ�� ________________________________________��
��5����ҺD�и����ӵ�Ũ���ɴ�С��˳��Ϊ��__________________________________
��6����ҵ����X��F�Ƶ�CuSO4��������ʹ�õ���ǡ�����Լ�������_____��_________��
| A��ŨH2SO4 | B��Fe2O3 | C��HNO3 | D��O2 E.H2O2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�ڽ���ұ���͵��ӹ�ҵ�У��Ƴ���Ϊ�����ij��������Գ�ȥ���ĵ�������һ��ѧ��ȤС��Խ������ڿ�����ȼ�յIJ�����������̽��ʵ�顣ȡһ�����ĸ��ڿ����м��ȣ�ʹ����ȼ�գ�ȼ��ʱ�����ש��ɫ��ȼ�պ�ð�ɫ����M����M��ϸ���ȡ4��84g������ƿB�У��μ�����ˮʹ������ȫ��Ӧ��ͨ����ͼ�������ɵ�������������������ɫ����ijɷ֡�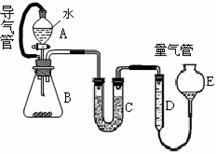
��1���ýྻ��˿պȡCaCl2��Һ����ɫ���������գ�����Ϊ__________������A��B�ĵ����ܵ�������___________________________��C�����ӿ���״������_______________��
��2��������D��E��Ӧ�ӵ�Һ����_________��
A��ˮ B�����Ȼ�̼ C���Ҵ� D������
��3��ʵ���У�Bƿ�ڷ��ȡ�B�з�Ӧ������ȡ���������������ʱ����ȷ�IJ���Ϊ______________________��
��4����ȡD���������ʱ��B��C�����������������壬�Ƿ�Ӱ��Ӧ�ò�õ��������__________________��
��5������D�еõ�����448mL���ѻ���Ϊ��������������д̼�����ζ���ܹ�ʹʪ��ĺ�ɫʯ����ֽ��������4��84g����M�еijɷ֣��ѧʽ����������Ϊ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���к�CaO���ʵ�CaC2������ij�о���ѧϰС���ͬѧ�����������ַ����ⶨCaC2�����Ĵ��ȡ�����д���пհף�
��1����һ�ַ����������ͼ��ѡ���ʵ���װ�ã����һ��ʵ�飬�ⶨCaC2�����Ĵ��ȡ�
��ѡ��װ�õ�����˳��Ϊ������ӿڵ���ĸ���� ��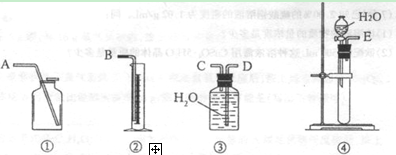
��2���ڶ��ַ���������������ˮ����ƿ�з�Ӧǰ�������ı仯���ⶨCaC2�������������ȳ�ȡ����1.50g����������ƿ��ˮ������Ϊ195.00g���ٽ�����������ƿ�У���Ӧ������ÿ����ͬʱ���õ��������±���
| | �������� | ����/g |
| ��ƿ��ˮ������ | ��1�� | 196.30 |
| ��2�� | 196.15 | |
| ��3�� | 196.05 | |
| ��4�� | 196.00 | |
| ��5�� | 196.00 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��һ��(4��)�����е����������ʣ�д����ȥ��Щ���ʵ��Լ���
��1��MgO (Al2O3) ��2��Cl2(HCl)
��3��FeCl3(FeCl2) ��4��NaHCO3��Һ(Na2CO3)
������(6��)��ˮ�к��д������Ȼ�þ���Ӻ�ˮ����ȡþ��������������ͼ��ʾ��
�ش��������⣺
д���ں�ˮ�м�������������������þ�Ļ�ѧ����ʽ ��
��������Ҫ��ָ ���Լ��ٿ�ѡ�� ��
��������ָ �������������տɵý���þ��
��������8�֣�ʵ��������480ml 0��1mol��L-1��Na2CO3��Һ���ش��������⣺
��1��Ӧ��������ƽ��ȡʮˮ̼���ƾ��� g��
��2����ͼ��ʾ������������Һ�϶�����Ҫ���� (�����)����ʵ�����貣������E���Ϊ mL��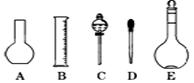
��3������ƿ�ϱ��У����¶ȡ���Ũ�ȡ�����������ѹǿ���ݿ̶��ߡ�����ʽ���ʽ�������е� ���������ַ��ţ�
��4�������������Ҫ�����ǣ�a����ƿ��b�ձ���c��ͷ�ιܡ�d������ƽ�������ڲ���������ʹ�õ�ǰ��˳���� ������д��ĸ��ÿ������ֻ��ѡ��һ�Σ�
��5���������ǻ�ѧʵ���г��õ�һ�ֲ������ߣ�����������Һ�Ĺ����в����������� ����;������д���֣�
��6����ʵ��ʱ���������������ʹ��Һ��Ũ��ƫ�͵��� ��
| A������ǰû�н�����ƿ�е�ˮ������ |
| B��̼����ʧȥ�˲��ֽᾧˮ�� |
| C��̼���ƾ��岻�������л����Ȼ��ƣ� |
| D������̼���ƾ���ʱ�����������⣻ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijУ��ѧС���ͬѧ��һ����������·������õ���Cu��Al��Fe������Au��Pt�Ƚ����Ļ���������������Ʊ�����ͭ������������ķ�����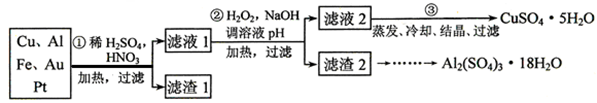
�ش��������⣺
��1���ڢٲ��μӷ�Ӧ�Ľ����� �֡�
��2���ڢڲ�����H2O2����Ϊ��Һ1�к��� ���ӡ�ʹ��H2O2���ŵ��� ��
��3�� �õڢ۲�����CuSO4��5H2O�Ʊ���ˮ����ͭ�ķ����ǣ� ��
��4���������ѧС���ͬѧ���������2��ȡAl2(SO4)3��18H2O ��ʵ�鲽�裺
��ȡ����2������������ ����ַ�Ӧ����ˣ�
��ȡ��Һ������������ ����д�Լ��Ļ�ѧʽ����Ȼ�� ����д����ʵ����������ƣ���
��3��������ϡ�����ܽ⣻
��4����� ����д����ʵ����������ƣ������Al2(SO4)3��18H2O���塣
��1���ڢ۲�����CuSO4��5H2O���п���������Na2SO4��Ϊ�����������ⶨCuSO4��5H2O�Ĵ��ȣ�ѡ��BaCl2(aq)��������Ҫ���Լ������г�����ⶨ�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����ʵ������д������
| A����������ʱ��Ӧʹ������е�ˮ����ȫ���ɺ���ֹͣ���� |
| B���������ʱ��Ӧʹ�¶ȼ�ˮ����������ƿ��֧�ܿڴ� |
| C����Һ����ʱ����Һ©���²�Һ��Ӧ���¿ڷų����ϲ�Һ��Ӧ���Ͽڵ��� |
| D����ȡ����ʱ����ѡ��ȡ���ܽ���������Ӧ����ԭ�ܼ�����ԭ�ܼ��������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com