



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���٢� | B���ڢ� | C���٢� | D���ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
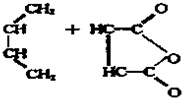
| �� |
| �� |
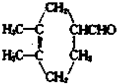
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
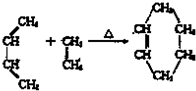
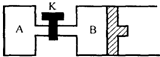 ��ͼ��ʾ��B�л����������ɻ�����A��B�о���1mol X��1mol Y����ʼʱ��V��A��=V��B��=a L���ر�K����ͬ�¶��£���������ͬʱ������Ӧ��2X��g��+2Y��g��=Z��g��+2W��g����H��0���ﵽƽ�⣨��ʱ��V��B��=0.8a L��
��ͼ��ʾ��B�л����������ɻ�����A��B�о���1mol X��1mol Y����ʼʱ��V��A��=V��B��=a L���ر�K����ͬ�¶��£���������ͬʱ������Ӧ��2X��g��+2Y��g��=Z��g��+2W��g����H��0���ﵽƽ�⣨��ʱ��V��B��=0.8a L���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��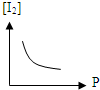 |
B��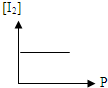 |
C��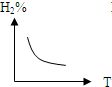 |
D��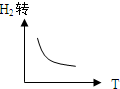 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

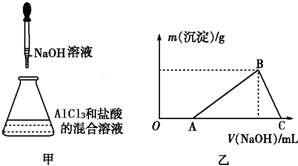
| A������������һ������Al������������ȷ�� |
| B������������һ��������AlCl3 |
| C������������һ������MgCl2��FeCl2 |
| D������������һ������ ��NH4��2SO4��MgCl2�������ʵ���֮��Ϊ1��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
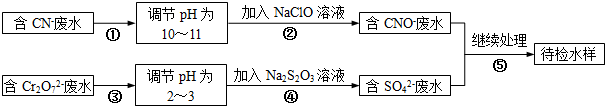
| A������[KAl��SO4��2?12H2O]��ˮ�����γ�Al��OH��3���壬��������ˮ�� |
| B��Na�Ľ������Ա�Mgǿ���ʿ���Na��MgCl2��Һ��Ӧ��Mg |
| C��ŨH2SO4��ǿ�����ԣ���������Cu������Ӧ |
| D��SO2���л�ԭ�ԣ��ʿ���Ư�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com