
 ����һ���Σ���A��B��C��D��E���ֶ�����Ԫ��Ԫ����ɣ�������ˮ��ɵ�����������ӣ����к�����A��B�γɵ�10���������ӣ�AԪ��ԭ�Ӻ�����������E����1��D��E����ͬ���壮�ü�������ʵ�飺
����һ���Σ���A��B��C��D��E���ֶ�����Ԫ��Ԫ����ɣ�������ˮ��ɵ�����������ӣ����к�����A��B�γɵ�10���������ӣ�AԪ��ԭ�Ӻ�����������E����1��D��E����ͬ���壮�ü�������ʵ�飺 ���ʴ�Ϊ����3���ڢ�A�壻
���ʴ�Ϊ����3���ڢ�A�壻 ��
��
| ||
| ||
| ||
| ||


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��0.15 mol?L-1 |
| B��0.2 mol?L-1 |
| C��0.3 mol?L-1 |
| D��0.4 mol?L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
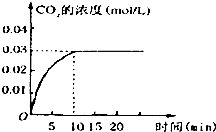 ���ݻ�Ϊ1L���ܱ������ڣ�����0.1molCO��0.1molH2O���ڴ������ڵ������¸��¼���ʹ�䷴Ӧ�����CO2��Ũ����ʱ��仯��ͼ����ͼ��
���ݻ�Ϊ1L���ܱ������ڣ�����0.1molCO��0.1molH2O���ڴ������ڵ������¸��¼���ʹ�䷴Ӧ�����CO2��Ũ����ʱ��仯��ͼ����ͼ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
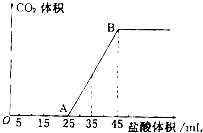 ʵ����������0.1mol/L NaOH��Һ�������й�����ʵ�飬��ݴ˻ش���������
ʵ����������0.1mol/L NaOH��Һ�������й�����ʵ�飬��ݴ˻ش����������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
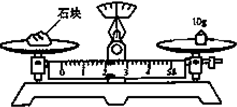 ��������10mol/L��ŨH2SO4���Ƴ�Ũ��Ϊ0.1mol/L�� 500mLϡH2SO4�IJ������밴Ҫ����գ�
��������10mol/L��ŨH2SO4���Ƴ�Ũ��Ϊ0.1mol/L�� 500mLϡH2SO4�IJ������밴Ҫ����գ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com