
±�ػ�ѧ�ḻ��ʣ����γ�±���±�ػ������±��������͵Ļ����
��д�������ӵ����ӽṹʾ��ͼ����������������������������������
��д��±�ػ��������ԭ�ӱ�1��1�Ĺ��ۻ����IBr���ĵ���ʽ�� �� ����������Ԫ�صĻ��ϼ�Ϊ���� ����
�Ƿ���������ķе�ϸߣ�������Ϊ���������֮�����������������
�Ȣ�HClO4����HClO����H2SO4��������ǿ������˳��Ϊ����������������ţ���
�������Ƶ���ˮ��NaBr��Һ�����Ȼ�̼��Ϊ�Լ�����֤�����Ļ�����ǿ���嵥�ʡ�����ʵ��������̺�ʵ�������������������������������������������� ����
 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
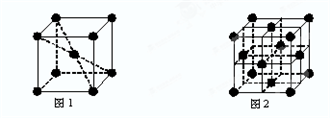
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
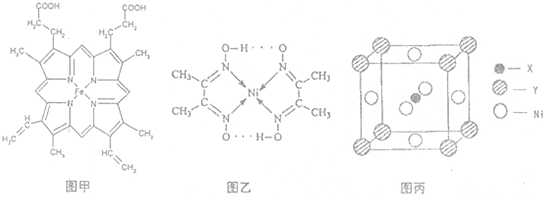
 ��YԪ��ԭ�ӵļ۵����Ų�ʽΪ3s2���þ����һ��������ͼ����ʾ����þ���Ļ�ѧʽΪ
��YԪ��ԭ�ӵļ۵����Ų�ʽΪ3s2���þ����һ��������ͼ����ʾ����þ���Ļ�ѧʽΪ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


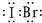
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com