
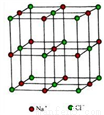
���ܵĴ洢������Ӧ�õ���Ҫƿ������λ�⻯��������廯������Ŀǰ�����õ���Ҫ������ϡ�
��1��Ti��BH4��2��һ�ֹ���Ԫ�����⻯�ﴢ����ϡ��ڻ�̬Ti�У��۵����Ų�ʽΪ ____���۵����Ų�ͼΪ____
��2��Һ���Ǹ������ʣ������ܵ��������壬����N2+3H2 2NH3,ʵ�ִ�������⡣����˵����ȷ����_____ ��
2NH3,ʵ�ִ�������⡣����˵����ȷ����_____ ��
a��NH3�����е�ԭ�ӵĹ���ӻ���ʽΪsp2�ӻ�
b��NH+4��PH+4��CH4��BH-4��ClO��4��Ϊ�ȵ�����
c����ͬѹǿʱ��NH3�ķе��PH3�ķе��
d��[Cu��NH3��4]2+�����У�Nԭ������λԭ��
��3���ü۲���ӶԻ��������ƶ�SnBr2�����У�Snԭ�ӵĹ���ӻ���ʽΪ__________��
SnBr2������ Sn-Br�ļ���______120��(�������������=��)��
(4) NiO �ľ���ṹ���Ȼ�����ͬ�� �ھ����������ӵ���λ����_______��
��֪�����ı߳�Ϊ a nm�� NiO ��Ħ������Ϊ b g��mol-1�� NAΪ�����ӵ�������ֵ�� ��NiO ������ܶ�Ϊ_________g��cm-3��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017�����ʡ���������е�ʮ��У�ص���ѧ������һ��������ѧ�Ծ��������棩 ���ͣ�ѡ����
�����£�Ksp(AgCl)=1.8��10-10, Ksp(AgI)=1.0��10-14,���������AgCl��AgI�ı�����Һ�����ϣ��������м���һ������AgNO3���壬����˵����ȷ����
A. ��AgI��Һ�м���AgNO3��c(Ag+)����Ksp(AgI)Ҳ����
B. ����Һ��ϣ�AgCl��AgI������
C. ��ȡ0.1435gAgCl�������100mLˮ����������仯����c(Cl-)Ϊ0.01mol/L
D. ��AgNO3������AgCl��AgI���ɳ���������AgClΪ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꽭��ʡ�߶�3��ѧҵˮƽ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��������ֻ������ˮ�����������ſ������ռ����ǣ� ��
A. Cl2 B. NO2 C. CO D. SO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����и����ڶ���ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ��ƶ���
4-����-3�����������Ǻϳ����ʪ�ؽ�������ҩ�ﰬ��Ī�µ���Ҫԭ�ϡ�4-����-3-���������ѵĺϳ�·�����£�
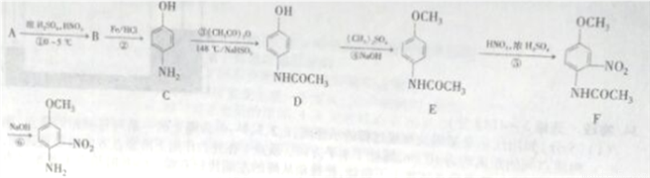
��֪��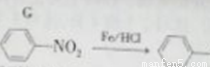 NH2���������ԣ��ױ�������
NH2���������ԣ��ױ�������
�ش��������⣺
(1)A�Ľṹ��ʽΪ_______________��B��������_______________��
(2)��Ӧ�ڵķ�Ӧ������____________��
(3)C�й����ŵ�������_________________��
(4)д����Ӧ�۵Ļ�ѧ����ʽ��________________����Ӧ���¶Ƚϸߣ�������NaHSO3�ᵼ�·�Ӧ�����ϵ����ɫ���Բ�ͬ�����ܵ�ԭ����________________��
(5)д����Ӧ�Ļ�ѧ����ʽ��______________________��
(6)H��E��ͬ���칹�壬���������������Ľṹ��____�֣������������칹�������к˴Ź�����������4��壬�ҷ������Ϊ1:2:2:6�Ľṹ��ʽΪ_____________��
�ٺ���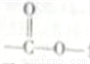 �ṹ���ܷ���������Ӧ
�ṹ���ܷ���������Ӧ
��Nԭ��ֱ���뱽���������ҽṹ�в�����N��O����
�۱�����������ȡ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����и����ڶ���ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
þȼ�ϵ����Ϊһ�ָ��ܻ�ѧ��Դ�����б������ߡ�ʹ�ð�ȫ���㡢�ɱ��͡�ȼ���������ˡ���ȾС���ص㣬ӵ�����õ�Ӧ��ǰ������ͼ��þȼ�ϵ�ص�һ��ԭ��ͼ����װ��ΪԲͲ״��������Ϊþ����ԲͲΪ�����ĵ�����ϡ������йظ�þȼ�ϵ�ص�������ȷ����
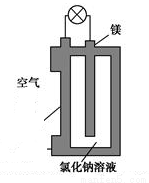
A. �õ�ص��ܷ�ӦΪ2Mg+O2=2MgO
B. ��Ӧ����O2-������������������
C. Cl-������ʧȥ��������Cl2
D. ������ӦʽΪO2+2H2O+4e-=4OH-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017���Ĵ�ʡ������ѧ�ڵ��Ĵ��¿������ۺϻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
����ͼʾ���Ӧ�������������

A. ��0.1000mol/LNaOH��Һ�ֱ�ζ�Ũ����ͬ������һԪ�ᣬ��ͼ4����ȷ���ٵ�������ǿ
B. ��0.0100mol/L����������Һ���ζ�Ũ�Ⱦ�Ϊ0.1000mol/LCl-��Br-��I-�Ļ����Һ����ͼ5���ߣ���ȷ�����ȳ�������Cl-
C. �������ͬ�������ܱ������У��ֱ������ͬ����O2��X���壬��ͼ6��ȷ��X������CH4����
D. ��ͼ7��˵��ϩ����H2�ӳɷ�Ӧ�Ƿ��ȷ�Ӧ�����߱�ʾ���д����������½���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�Ƹ��и���3��������������ۺϻ�ѧ�Ծ��������棩 ���ͣ��ƶ���
[��ѧ-ѡ��5���л���ѧ����]
��ˮ���ܸ߷���M������N������ͼ��ʾ;���ϳ�(���ֲ�����ȥ����
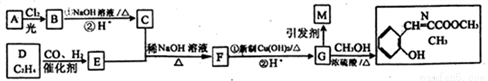
��֪��
��A�Ƿ������ĺ����������Է�������Ϊ108
���л�������У�ͬһ��̼ԭ���������������ǻ�ʱ���ȶ������Զ���ˮ
(S)R1CHO + R2CH2CHO R1CH=
R1CH=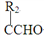 +H2O
+H2O
��ش��������⣺
(1)E�Ļ�ѧ����_______��C�Ľṹ��ʽ _______��
(2) M�еĺ��������ŵ�����Ϊ_______��A����B�ķ�Ӧ����________��
(3)д��F��G�ĵ�һ����ѧ����ʽ______________��
(4)ͬʱ��������������G��ͬ���칹����____�֣������������칹������G������
�ٱ����������ֲ�ͬ����
������NaHCO3��Һ��Ӧ��������
�����Ȼ�����Һ����ɫ
�ܷ�����ֻ����һ����
(5)�������еĺϳ���·ͼ�����Ҵ�Ϊ��ʼ����Լ���ѡ��д���ϳɾ�-2-��ϩȩ����·ͼ��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ��ɳ�г���������ظ���3��ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʵ���������ʵ�����������Ӱ�����
A. ������ˮʪ��pH��ֽ��ⶨ��������Һ��pH
B. �ⶨ�кͷ�Ӧ�ķ�Ӧ��ʱ��������Һ������������Һ��
C. ������к͵ζ����ⶨδ֪Ũ�ȵļ�Һʱ������ƿ�м���2��3mL��̪��Һ��ָʾ��
D. �ڵ�����Һ�м���ϡ�������һ��ʱ����ٵμ�������Һ������۵�ˮ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�����и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��һ����ɱ���ܱ������У�����һ������X��Y��Z��������ӦmX(g)+nY(g) pZ(g) ��H=Q kJ/mol����Ӧ�ﵽƽ���Y�����ʵ���Ũ�����¶ȡ���������Ĺ�ϵ���±���ʾ��
pZ(g) ��H=Q kJ/mol����Ӧ�ﵽƽ���Y�����ʵ���Ũ�����¶ȡ���������Ĺ�ϵ���±���ʾ��
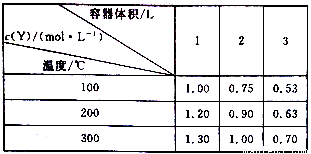
����˵������ȷ����
A. m+n>p
B. Q <0
C. ������䣬�¶����ߣ�ƽ�����淴Ӧ�����ƶ�
D. �¶Ȳ��䣬ѹǿ����Y��������������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com