��2012?������ģ����ν��ʹ�����CO
2�ĺ�������Ч�ؿ�������CO
2������ȫ������ձ����ӣ�Ϊ��С������CO
2�Ի�����Ӱ�죬һ����������������������ŷ�������һ�����ѧ�Ҽ�ǿ�˶�CO
2�������õ��о���
��1��Ŀǰ��ҵ����һ�ַ�������CO
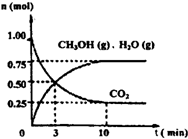
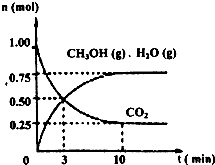
2������ȼ�ϼ״���Ϊ̽���÷�Ӧԭ������������ʵ�飺ij�¶��£����ݻ�Ϊ2L���ܱ������У�����1mol CO
2��3.25mol H
2��һ�������·�����Ӧ�����CO
2��CH
3OH��g����H
2O��g�������ʵ�����n����ʱ��仯����ͼ��ʾ��
�ٴӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ����v��H
2��=
0.1125mol/��L?min��
0.1125mol/��L?min��
��
�����д�ʩ��һ������ʹCO
2��ת�����������
ACD
ACD
��
A����ԭ�������ٳ���1mol CO
2 B����ԭ�������ٳ���1mol H
2C����ԭ�����г���1mol���� D��ʹ�ø���Ч�Ĵ���
E����С�������ݻ� F����ˮ��������ϵ�з���
��2�����³�ѹ�£�����CO
2ˮ��Һ��pH=5.6��c��H
2CO
3��=1.5��10
-5mol/L��������ˮ�ĵ��뼰H
2CO
3�ĵڶ������룬��H
2CO
3?HCO
3-+H
+�ĵ���ƽ�ⳣ��K=
4.2��10-7
4.2��10-7
������֪��10
-5.6=2.5��10
-6����
��3����״���£���4.48L CO
2ͨ��200mL 1.5mol/L��NaOH��Һ��������Һ������Ũ���ɴ�С��˳��Ϊ
c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+��
c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+��
��
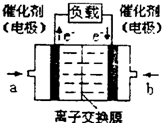
��4����ͼ�Ǽ״�ȼ�ϵ�أ��������ҺΪKOH��Һ���Ľṹʾ��ͼ����a��ͨ�����
�״�
�״�
����״�����������������缫�Ϸ����ĵ缫��ӦʽΪ
CH3OH+8OH--6e-�TCO32-+6H2O
CH3OH+8OH--6e-�TCO32-+6H2O
��
��5����֪��������K
sp��AgCl��=2.0��10
-10��K
sp��AgBr��=5.4��10
-13����AgNO
3��Һ�м���KBr��KCl�������ֳ�������ʱ����Һ��c��Br
-����c��Cl
-���ı�ֵΪ
2.7��10-3
2.7��10-3
��




 2009��12��7��һ18���ڵ������籾�����ٿ������Ϲ�������飬��δ��Ӧ������仯��ȫ���ж�ǩ���µ�Э�飮����ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2��������ȫ������ձ����ӣ�Ϊ��С������CO2�Ի�����Ӱ�죬һ����������������������ŷ�������һ�����ѧ�Ҽ�ǿ�˶�CO2�������õ��о���
2009��12��7��һ18���ڵ������籾�����ٿ������Ϲ�������飬��δ��Ӧ������仯��ȫ���ж�ǩ���µ�Э�飮����ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2��������ȫ������ձ����ӣ�Ϊ��С������CO2�Ի�����Ӱ�죬һ����������������������ŷ�������һ�����ѧ�Ҽ�ǿ�˶�CO2�������õ��о���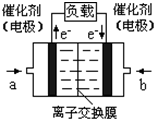 ��ͼ����a��ͨ�����
��ͼ����a��ͨ����� ��2012?������ģ����ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2������ȫ������ձ����ӣ�Ϊ��С������CO2�Ի�����Ӱ�죬һ����������������������ŷ�������һ�����ѧ�Ҽ�ǿ�˶�CO2�������õ��о���
��2012?������ģ����ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2������ȫ������ձ����ӣ�Ϊ��С������CO2�Ի�����Ӱ�죬һ����������������������ŷ�������һ�����ѧ�Ҽ�ǿ�˶�CO2�������õ��о���