
 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д� ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

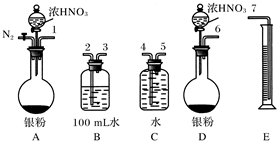
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �� |
| �� |
| Ũ���� |
| �� |
| Ũ���� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ѧϰ���ֱ���һ��ѧ�˽̰� �˽̰� ���ͣ�022
ij����С�飬�ռ���һ�ֺϽ�����о���
(1)��۰���ɫ������⻬��
(2)�ھƾ��������գ�������ɫ���Ͻ�Ƭ�ۻ����������䣻
(3)ȡ��ȥ����Ľ���10.0 g����������H2SO4�У��ռ�����״���µ�H2��9.96 L��
(4)��ȡ��ȥ��Ƥ�Ľ���10.0 g����������NaOH(aq)�У�Ҳ�ռ�����״���µ�H2��9.96 L��
�Ծݴ��жϣ��Ͻ���һ�����е�Ԫ����________(дԪ�ط���)������Ԫ�ؿ��ܺ��е���________(��ѡ����)��
a��Ag����b��Mg����c��Na����d��Fe
����úϽ��У�ֻ��2��Ԫ�أ����ǵ���������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����ѧ����ñ���һ��ѧ�˽̰� �˽̰� ���ͣ�022
ij����С�飬�ռ���һ�ֺϽ�����о���
(1)��۰���ɫ����Ƥ�⻬��
(2)�ھƾ��������գ��������ɫ���Ͻ�Ƭ�ۻ����������䣮
(3)ȡ��ȥ��Ƥ�Ľ���10.0 g����������H2SO4�У��ռ�����״���µ�H2��9.96 L��
(4)��ȡ��ȥ��Ƥ�Ľ���10.0 g����������NaOH(aq)�У�Ҳ�ռ�����״���µ�H2��9.96 L��
�Ծݴ��жϣ��Ͻ���һ�����е�Ԫ����________(дԪ�ط���)������Ԫ�ؿ��ܺ��е���________(��ѡ����)��
A��Ag
B��Mg
C��Fe
����úϽ��У�ֻ������Ԫ�أ����ǵ���������________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com