
(14��)(2011�����ݸ߶����)��25 ��ʱ����ʯī�缫���2.0 L 0.5 mol��L��1 CuSO4��Һ��5 min����һ��ʯī�缫����6.4 g Cu���ɡ��Իش��������⣺
(1)����������Ӧ����________�����缫��ӦʽΪ____________________________
________________________________________________________________________��
(2)��������Һ��������䣬�������Һ��pHΪ________��
(3)������Һ�ָ�������ǰһ�����������________mol��________��
(4)���õ�����������ͭƬ����ʯī���缫��������ͭƬ���������________g�����Һ��pH________��(���С����������䡱)
(1)����4OH����4e��===2H2O��O2��
(2)1��(3)0.1��CuO��(4)12.8������
��������(1)n(CuSO4)��2.0 L��0.5 mol/L��1.0 mol����������������CuΪ��0.1 mol����CuSO4δ��ȫ��⣬��������������Ӧ���缫��ӦΪ4OH����4e��===2H2O��O2����
(2)�ܷ�ӦΪ��
2CuSO4��2H2O2Cu������O2��������2H2SO4
2 2 2 1 2
0.1 mol 0.1 mol 0.1 mol 0.05 mol 0.1 mol
���Ե���c(H��)����0.1 mol/L��
��pH����lg 0.1��1��
(3)�������ɵ�0.1 mol Cu��0.05 mol O2�������ϵ�����൱��0.1 mol CuO�����������Һ��ԭ����Ӧ����0.1 mol CuO��
(4)��ʱ��Ϊ��Ƴأ�����Cu��2e��===Cu2����������Cu2����2e��===Cu�����������������6.4 gͭ���������ܽ�6.4 gͭ��������ͭƬ������Ϊ6.4 g��6.4 g��12.8 g�������Һ��pH���䡣


 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| (2)H+ |
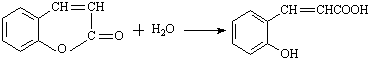
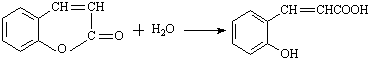
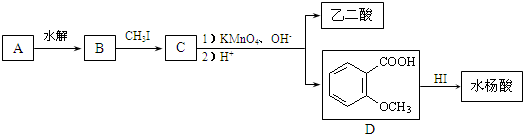
 CH2CH2
CH2CH2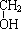 ����Ӧ�ϳ�PBSA�Ļ�ѧ����ʽ��
����Ӧ�ϳ�PBSA�Ļ�ѧ����ʽ��| һ������ |
 OC-COO��CH2��4O
OC-COO��CH2��4O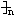 H+��2n-1��H2O
H+��2n-1��H2O| һ������ |
 OC-COO��CH2��4O
OC-COO��CH2��4O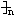 H+��2n-1��H2O
H+��2n-1��H2O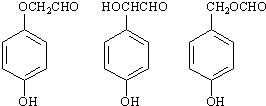

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
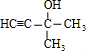
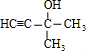
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
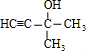
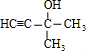
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�갲��ʡ���������ѧ�߶���ѧ����������3��ѧ�Ծ����������� ���ͣ������
(14��)(2011�����ݸ߶����)��25 ��ʱ����ʯī�缫���2.0 L 0.5 mol��L��1 CuSO4��Һ��5 min����һ��ʯī�缫����6.4 g Cu���ɡ��Իش��������⣺
(1)����������Ӧ����________�����缫��ӦʽΪ____________________________
________________________________________________________________________��
(2)��������Һ��������䣬�������Һ��pHΪ________��
(3)������Һ�ָ�������ǰһ�����������________mol��________��
(4)���õ�����������ͭƬ����ʯī���缫��������ͭƬ���������________g�����Һ��pH________��(���С����������䡱)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com