
 CH3OH��H2O����ü״������۲����뷴Ӧ�¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ����ش��������⣺
CH3OH��H2O����ü״������۲����뷴Ӧ�¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ����ش��������⣺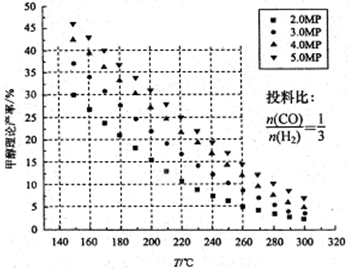
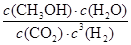 ��
��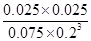 ��1.04 ��3�֣�
��1.04 ��3�֣�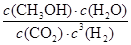 ��
��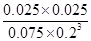 ��1.04
��1.04

 ���ٴ�����ɽ����ϵ�д�
���ٴ�����ɽ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������


 CH3OH(g)��CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ����
CH3OH(g)��CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ����
 CH3OH(g)��Ӧ��ƽ�ⳣ��Ϊ________(�ú�a��V�Ĵ���ʽ��ʾ)��
CH3OH(g)��Ӧ��ƽ�ⳣ��Ϊ________(�ú�a��V�Ĵ���ʽ��ʾ)��
 CH3OH(g)��H2O(g)�����CO2(g)��CH3OH(g)��Ũ����ʱ��仯��������ͼ��ʾ��
CH3OH(g)��H2O(g)�����CO2(g)��CH3OH(g)��Ũ����ʱ��仯��������ͼ��ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��2HI(g) =H2(g)��I2(g)?H��+11 kJ��mol |
| B��H2(g)��I2(g) =2HI(g)?H����22 kJ��mol |
| C��H2(g)��I2(g) =2HI(g)?H��+288 kJ��mol |
| D��H2(g)�� I2(g) =2HI(g)?H����144 kJ��mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2SO3(g) ��H����196.6 kJ��mol��1)����ش��������⣺
2SO3(g) ��H����196.6 kJ��mol��1)����ش��������⣺
 CH3COOC2H5(l)��H2O(l) ��H����8.62 kJ��mol��1
CH3COOC2H5(l)��H2O(l) ��H����8.62 kJ��mol��1
 H2(g)��CO2(g)ƽ�ⳣ�����¶ȵı仯���±���
H2(g)��CO2(g)ƽ�ⳣ�����¶ȵı仯���±���| �¶�/�� | 400 | 500 | 800 |
| ƽ�ⳣ��K | 9.94 | 9 | 1 |
| | n(CO) | n(H2O) | n(H2) | n(CO2) |
| A | 1 | 5 | 2 | 3 |
| B | 2 | 2 | 1 | 1 |
| C | 3 | 3 | 0 | 0 |
| D | 0.5 | 2 | 1 | 1 |
| E | 3 | 1 | 2 | 1 |
 2CO(g)ƽ�ⳣ��ΪK��
2CO(g)ƽ�ⳣ��ΪK�� CO(g)��H2(g) ƽ�ⳣ��ΪK1��
CO(g)��H2(g) ƽ�ⳣ��ΪK1�� H2(g)��CO2(g) ƽ�ⳣ��ΪK2��
H2(g)��CO2(g) ƽ�ⳣ��ΪK2���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

 CO2(g); ��H=��395.5 kJ��mol-1
CO2(g); ��H=��395.5 kJ��mol-1 2CO2(g); ��H=��560 kJ��mol-1
2CO2(g); ��H=��560 kJ��mol-1 TiCl4(s)+2CO(g)�Ħ�H=�D80kJ/mol
TiCl4(s)+2CO(g)�Ħ�H=�D80kJ/mol �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 CH3OH(g)+H2O(g) ��H����49.0 kJ/mol��
CH3OH(g)+H2O(g) ��H����49.0 kJ/mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 O2(g)�TCO2(g) ��H����283.0kJ/mol
O2(g)�TCO2(g) ��H����283.0kJ/mol CO2(g)��H2(g) ��H��0�ں����ܱ������У���ʼʱn(H2O)=0.20mol��n(CO)��0.10 mol,��8000Cʱ�ﵽƽ��״̬��K��1.0����ƽ��ʱ��������CO��ת������_____________(����������һλС��)��
CO2(g)��H2(g) ��H��0�ں����ܱ������У���ʼʱn(H2O)=0.20mol��n(CO)��0.10 mol,��8000Cʱ�ﵽƽ��״̬��K��1.0����ƽ��ʱ��������CO��ת������_____________(����������һλС��)�� 2NH3(g)����H��0���ڵ��������½��У��ı�������Ӧ��������I��II��III����ϵ�и�����Ũ����ʱ��仯����������ͼ��ʾ��
2NH3(g)����H��0���ڵ��������½��У��ı�������Ӧ��������I��II��III����ϵ�и�����Ũ����ʱ��仯����������ͼ��ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ��ѧ�� | N��N | H��H | N��H |
| ����kJ��mol��1 | 946 | 436 | 390 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com