
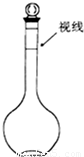
 �������������ʵ����ʵ��������Һ�������Ӱ���жϣ�
�������������ʵ����ʵ��������Һ�������Ӱ���жϣ�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ʵ������Ҫ����400mL0.2mol/L����������Һ��ʵ�鲽������У�
ʵ������Ҫ����400mL0.2mol/L����������Һ��ʵ�鲽������У��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��9�֣�(1)����д���пհ״���ʵ������Ҫ����500mL0.2mol/L����������Һ��ʵ�鲽������У�
A������ƽ�ϳƳ�___________g�����ƹ��壬���������ձ��������������ˮ�ܽ⡣
B���ѵõ�����ҺС�ĵ�����__________ע��________mL������ƿ�С�
C������������ˮϴ���ձ��Ͳ�����2��3�Σ�ÿ��ϴ��ҺҲС��ת������ƿ�С�
D������������ƿ�м�����ˮ��Һ���̶�l��2cm��������_______________С�ĵμ�����ˮ����Һ��Һ��ײ���̶���ˮƽ���С�
E����ƿ�����������ҡ�ȡ�
F������õ���Һ�����Լ�ƿ�У����ϱ�ǩ����ϴ������ƿ��
(2) ���������ʹ������ҺŨ��ƫ�ߵ���___________(�����)��
a��ijͬѧ����ʱ�۲�Һ����������ͼ��ʾ
b��û�н��������IJ�������C
c��������ˮʱ�����������˿̶���
d��������������
e������ƿ��ǰ�ڱ�մ��ˮ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��2011ѧ�꽭��ʡ�����к�ɽ�Ÿ���ѧ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��9�֣�(1)����д���пհ״���ʵ������Ҫ����500mL0.2mol/L����������Һ��ʵ�鲽������У�
| A������ƽ�ϳƳ�___________g�����ƹ��壬���������ձ��������������ˮ�ܽ⡣ |
| B���ѵõ�����ҺС�ĵ�����__________ע��________mL������ƿ�С� |
| C������������ˮϴ���ձ��Ͳ�����2��3�Σ�ÿ��ϴ��ҺҲС��ת������ƿ�С� |
| D������������ƿ�м�����ˮ��Һ���̶�l��2cm��������_______________С�ĵμ�����ˮ����Һ��Һ��ײ���̶���ˮƽ���С� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��㶫ʼ���ط����ѧ��һ��ѧ�����п������ۻ�ѧ�Ծ����������� ���ͣ�ʵ����
��16�֣���1������д���пհ״���ʵ������Ҫ����500mL0.2mol/L����������Һ��ʵ�鲽������У�
| A������ƽ�ϳƳ�___________g�����ƹ��壬���������ձ��������������ˮ�ܽ⡣ |
| B���ѵõ�����ҺС�ĵ�����__________ע��________mL������ƿ�С� |
| C������������ˮϴ���ձ��Ͳ�����2��3�Σ�ÿ��ϴ��ҺҲС��ת������ƿ�С� |
| D������������ƿ�м�����ˮ��Һ���̶���������_______________С�ĵμ�����ˮ����Һ��Һ��ײ���̶���ˮƽ���С� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ�����и߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��9�֣�(1)����д���пհ״���ʵ������Ҫ����500mL0.2mol/L����������Һ��ʵ�鲽������У�
A������ƽ�ϳƳ�___________g�����ƹ��壬���������ձ��������������ˮ�ܽ⡣
B���ѵõ�����ҺС�ĵ�����__________ע��________mL������ƿ�С�
C������������ˮϴ���ձ��Ͳ�����2��3�Σ�ÿ��ϴ��ҺҲС��ת������ƿ�С�
D������������ƿ�м�����ˮ��Һ���̶�l��2cm��������_______________С�ĵμ�����ˮ����Һ��Һ��ײ���̶���ˮƽ���С�
E����ƿ�����������ҡ�ȡ�
F������õ���Һ�����Լ�ƿ�У����ϱ�ǩ����ϴ������ƿ��
(2) ���������ʹ������ҺŨ��ƫ�ߵ���___________(�����)��

a��ijͬѧ����ʱ�۲�Һ����������ͼ��ʾ
b��û�н��������IJ�������C
c��������ˮʱ�����������˿̶���
d��������������
e������ƿ��ǰ�ڱ�մ��ˮ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com