
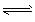 H2 (g)+I2 (g)ŅŃ“ļµ½Ę½ŗāµÄŹĒ
H2 (g)+I2 (g)ŅŃ“ļµ½Ę½ŗāµÄŹĒ  Ó¢ÓļŠ”Ó¢ŠŪĢģĢģĬŠ“ĻµĮŠ“š°ø
Ó¢ÓļŠ”Ó¢ŠŪĢģĢģĬŠ“ĻµĮŠ“š°ø Źī¼Ł×÷Ņµ°²»ÕÉŁÄź¶łĶƳö°ęÉēĻµĮŠ“š°ø
Źī¼Ł×÷Ņµ°²»ÕÉŁÄź¶łĶƳö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĖµ·ØÖŠæÉŅŌĖµĆ÷2HI£Øg£©![]() H2£Øg£©+I2£Øg£©ŅŃ“ļµ½Ę½ŗāדĢ¬µÄŹĒ£Ø £©
H2£Øg£©+I2£Øg£©ŅŃ“ļµ½Ę½ŗāדĢ¬µÄŹĒ£Ø £©
£Ø1£©µ„Ī»Ź±¼äÄŚÉś³Én mol H2µÄĶ¬Ź±Éś³Én mol HI
£Ø2£©Ņ»øöH”ŖH¼ü¶ĻĮѵÄĶ¬Ź±ÓŠĮ½øöH”ŖI¼ü¶ĻĮŃ
£Ø3£©c(HI)= c(I2)
£Ø4£©·“Ó¦ĖŁĀŹ¦Ō(H2)=¦Ō(I2)=0.5¦Ō(HI)
£Ø5£©c(H2)”Ćc(I2)”Ćc(HI)=2”Ć1”Ć1
£Ø6£©ĪĀ¶ČŗĶĢå»żŅ»¶ØŹ±£¬Ä³Ņ»Éś³ÉĪļÅØ¶Č²»ŌŁ±ä»Æ
£Ø7£©ĪĀ¶ČŗĶĢå»żŅ»¶ØŹ±£¬ČŻĘ÷ÄŚŃ¹Ēæ²»ŌŁ±ä»Æ
£Ø8£©Ģõ¼žŅ»¶Ø£¬»ģŗĻĘųĢåµÄĘ½¾łĻą¶Ō·Ö×ÓÖŹĮæ²»ŌŁ±ä»Æ
£Ø9£©ĪĀ¶ČŗĶĢå»żŅ»¶ØŹ±£¬»ģŗĻĘųĢåµÄŃÕÉ«²»ŌŁ·¢Éś±ä»Æ
£Ø10£©ĪĀ¶ČŗĶŃ¹ĒæŅ»¶ØŹ±£¬»ģŗĻĘųĢåµÄĆÜ¶Č²»ŌŁ·¢Éś±ä»Æ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĖµ·ØÖŠæÉŅŌĖµĆ÷2HI![]() H2+I2£Øg£©ŅŃ“ļµ½Ę½ŗāדĢ¬µÄŹĒ_____ __
H2+I2£Øg£©ŅŃ“ļµ½Ę½ŗāדĢ¬µÄŹĒ_____ __
¢Åµ„Ī»Ź±¼äÄŚÉś³Én mol H2µÄĶ¬Ź±Éś³Én mol HI£»
¢ĘŅ»øöH”ŖH¼ü¶ĻĮѵÄĶ¬Ź±ÓŠĮ½øöH”ŖI¼ü¶ĻĮŃ£»
¢Ēc(HI)= c(I2)£»
¢Č·“Ó¦ĖŁĀŹ¦Ō(H2)=¦Ō(I2)=0.5¦Ō(HI)£»
¢Éc(H2)”Ćc(I2)”Ćc(HI)=2”Ć1”Ć1£»
¢ŹĪĀ¶ČŗĶĢå»żŅ»¶ØŹ±£¬Ä³Ņ»Éś³ÉĪļÅØ¶Č²»ŌŁ±ä»Æ£»
¢ĖĪĀ¶ČŗĶĢå»żŅ»¶ØŹ±£¬ČŻĘ÷ÄŚŃ¹Ēæ²»ŌŁ±ä»Æ£»
¢ĢĢõ¼žŅ»¶Ø£¬»ģŗĻĘųĢåµÄĘ½¾łĻą¶Ō·Ö×ÓÖŹĮæ²»ŌŁ±ä»Æ£»
¢ĶĪĀ¶ČŗĶĢå»żŅ»¶ØŹ±£¬»ģŗĻĘųĢåµÄŃÕÉ«²»ŌŁ·¢Éś±ä»Æ£»
¢ĪĪĀ¶ČŗĶŃ¹ĒæŅ»¶ØŹ±£¬»ģŗĻĘųĢåµÄĆÜ¶Č²»ŌŁ·¢Éś±ä»Æ£»
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ±±¾©ĘŚÄ©Ģā ĢāŠĶ£ŗµ„Ń”Ģā
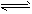 2C£Øg£©£¬ĻĀĮŠĖµ·ØÖŠæÉŅŌĖµĆ÷Õāøö·“Ó¦ŅŃ¾“ļµ½Ę½ŗāדĢ¬µÄŹĒ
2C£Øg£©£¬ĻĀĮŠĖµ·ØÖŠæÉŅŌĖµĆ÷Õāøö·“Ó¦ŅŃ¾“ļµ½Ę½ŗāדĢ¬µÄŹĒ ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗŗÓ±±Ź”ĘŚÄ©Ģā ĢāŠĶ£ŗ²»¶ØĻīŃ”ŌńĢā
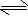 2NH3ŅŃ“ļĘ½ŗāµÄŹĒ
2NH3ŅŃ“ļĘ½ŗāµÄŹĒ²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com