
 ���ش��������⣺
���ش��������⣺
 ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
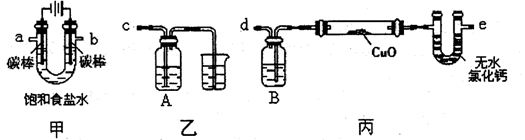
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
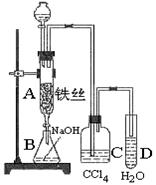
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ʵ���� | T/K | ����������/ g | KMnO4������Һ��Ũ��/mol?L-1 | ʵ��Ŀ�� |
| �� | 298 | 0.5 | 0.01 | ����ʵ��ٺ͢�̽��KMnO4������Һ��Ũ�ȶԸ÷�Ӧ���ʵ�Ӱ�죻 ����ʵ��ٺ͢�̽���¶ȶԸ÷�Ӧ���ʵ�Ӱ�죻 ����ʵ��ٺ�______̽�������Ը÷�Ӧ���ʵ�Ӱ�� |
| �� | | | | |
| �� | | | | |
| �� | | 0 | |
| KMnO4������Һ ��Ũ�� / mol?L-1 | ��Һ��ɫ����ʱ�� t / min | ||
| ��1�� | ��2�� | ��3�� | |
| 0.01 | 14 | 13 | 11 |
| 0.001 | 6 | 7 | 7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
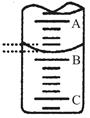
| A��c��Na+����c��CH3COO���� | B��c��Na+����c��CH3COO���� |
| C��c��Na+��=c��CH3COO���� | D������ȷ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������1 mLNaCl��Һ�������Թܣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿ� |
| B������1 mLNaCl��Һ�������Թܣ���ʪ�����ɫʯ����ֽ�����Թܿ� |
| C������1 mLNaOH��Һ�������Թܣ�������ĺ�ɫʯ����ֽ�����Թܿ� |
| D������1 mLNaOH��Һ�������Թܣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
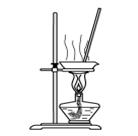
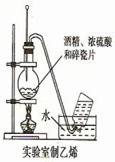


| A����ͼ1��ʾװ������NH4Cl������Һ�Ʊ�NH4Cl���� |
| B����ͼ2��ʾװ����ȡ������ϩ���� |
| C����ͼ3��ʾװ�÷���CCl4��ȡ��ˮ���ѷֲ���л����ˮ�� |
| D����ͼ4��ʾװ����ȡ����Cl2���� |
�鿴�𰸺ͽ���>>
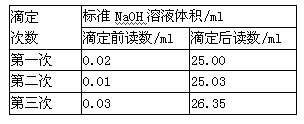
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| �ζ����� | ����Һ�����mL�� | �����������mL�� | |
| �ζ�ǰ������mL�� | �ζ��������mL�� | ||
| ��һ�� | 10.00 | 0.50 | 20.40 |
| �ڶ��� | 10.00 | 4.00 | 24.10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com