

���� ��1������M��Z�����ԭ������֮��Ϊ3��5������M��Z�����ԭ�������ֱ�Ϊ3x��5x��
����MCO3•ZCO3��MCO3��ZCO3�ı�ֵΪ1��1���ʵõ���������MO��ZO�����ʵ���֮��ҲΪ1��1������MCO3•ZCO3������Ϊ1.84g���õ������������Ϊ0.96g���ɵã�
$\frac{3x+5x+32}{3x+5x+120}$=$\frac{0.96}{1.84}$�����ɽ��xֵ���Ӷ��ó�M��Z�����ԭ�����������ó���ʯ�Ļ�ѧʽ��
��2�����������պ�IJ���ΪCaO��MgO�Ļ�������ո����������õ��ʹ軹ԭ�����õ�����Mg��һ�ֺ������Σ��ݴ�д����ѧ����ʽ��
������������ԭ��Ӧ�������ӵ�������ǿ��þ���ӣ����Ե���Ȼ�þ��ҺӦ�������ӷŵ磬���ò���þ���ʣ�
��3������ͼ����ͼl��֪�������¶����ߣ�K1�������H1��0��ϸ�˹���ɷ������
��4����ΪK3���¶ȵı仯ͼ��֪���¶�Խ��K3ԽС˵������Ӧ�Ƿ��ȷ�Ӧ���ٶ�tʱ��Ѹ�ٽ��µ�T2��ƽ�������ƶ����״���Ũ�ȱ���������µ��¶������´ﵽƽ�⣬�ɴ���ͼ��
��5������Ũ������ƽ�ⳣ���Ĵ�С��ϵ���жϷ�Ӧ���еķ���
��� �⣺��1������M��Z�����ԭ������֮��Ϊ3��5������M��Z�����ԭ�������ֱ�Ϊ3x��5x��
����MCO3•ZCO3��MCO3��ZCO3�����ʵ���֮��Ϊ1��1���ʵõ�����������MO��ZO�����ʵ���֮��ҲΪ1��1������MCO3•ZCO3������Ϊ1.84g���õ������������Ϊ0.96g���ɵã�
$\frac{3x+5x+32}{3x+5x+120}$=$\frac{0.96}{1.84}$��x=8��
M�����ԭ������Ϊ3x=24����MΪMg��
Z�����ԭ������Ϊ5x=40����ZΪCa�����ʯ�Ļ�ѧʽΪMgCO3•CaCO3���ʴ�Ϊ��MgCO3•CaCO3��
��2�����������պ�IJ���ΪCaO��MgO�Ļ�������ո����������õ��ʹ軹ԭ�����õ�����Mg��һ�ֺ������Σ����ڴ˺���������ֻ��Z��Si��OԪ�أ���Z��Si�����ʵ���֮��Ϊ2��1����ΪCa2SiO4���ʴ˷�Ӧ�Ļ�ѧ����ʽΪ��2MgO+2CaO+Si$\frac{\underline{\;����\;}}{\;}$Ca2SiO4+2Mg���ʴ�Ϊ��2MgO+2CaO+Si$\frac{\underline{\;����\;}}{\;}$Ca2SiO4+2Mg��
������������ԭ��Ӧ�������ӵ�������ǿ��þ���ӣ����Ե���Ȼ�þ��ҺӦ�������ӷŵ磬���ò���þ���ʣ��ʴ�Ϊ�����MgCl2��Һʱ��������H+��Mg2+���õ��ӣ��缫��Ӧʽ2H2O+2e��=H2��+2OH�������Բ��ܵõ�Mg���ʣ�
��3����Ӧ1��CO2��g��+H2��g��?CO��g��+H2O��g����H1
��Ӧ2��CO��g��+2H2��g��?CH3OH��g����H2
��Ӧ3��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H3
���ݸ�˹���ɣ���H1=��H3-��H2������ͼl��֪�������¶����ߣ�K1�������H1��0�����ԡ�H3-��H2��0������H3����H2���ʴ�Ϊ��С�ڣ���ͼl��֪�������¶����ߣ�K1�������H1��0�����ݸ�˹�����ֵá�H3=��H1+��H2�����ԡ�H2����H3��
��4����ΪK3���¶ȵı仯ͼ��֪���¶�Խ��K3ԽС˵������Ӧ�Ƿ��ȷ�Ӧ���ٶ�tʱ��Ѹ�ٽ��µ�T2��ƽ�������ƶ����״���Ũ�ȱ���������µ��¶������´ﵽƽ�⣬�����¶ȱ仯˲��Ũ����������û�м�ϣ�����ͼ��Ϊ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��5��һ���¶��´ﵽƽ����ü״���ת����Ϊ60%������ת���ļ״������ʵ���Ϊ0.12mol����ת����CO�����ʵ���Ϊ0.12mol����ƽ��ʱ�״������ʵ���Ϊ0.20mol-0.12mol=0.08mol��CO�����ʵ���Ϊ0.22mol-0.12mol=0.1mol����ƽ�ⳣ��K=$\frac{1}{c��C{H}_{3}OH��•c��CO��}$=$\frac{1}{\frac{0.08}{2}��\frac{0.1}{2}}$=500������Ϊ�״���ת����Ϊ60%����ת���ļ״������ʵ���Ϊ0.20mol��60%=0.12mol����������������ʵ���Ϊ0.12mol��ԭƽ����ϵ�������ܵ����ʵ���Ϊ0.08mol+0.1mol=0.18mol�����Ϊ2L��
ά���¶Ȳ��䣬�������ﵽƽ��ĺ�ѹ�����У�����˲��ͨ��0.12mol CH3OH��0.06mol CO������壬���������ܵ����ʵ���Ϊ0.12mol+0.06mol=0.18mol��ԭƽ����ȣ��������Ϊԭ����2������ʱ���״������ʵ���Ϊ0.08mol+0.12mol=0.2mol��CO�����ʵ���Ϊ0.1mol+0.06mol=0.16mol����Qc=$\frac{1}{\frac{0.2}{4}��\frac{0.16}{4}}$=500=K����ƽ�ⲻ�ƶ���
�ʴ�Ϊ�����ƶ�����������������ʵ�����ԭƽ������������ʵ�����ȣ������Ϊ4L��Qc=$\frac{1}{\frac{0.2}{4}��\frac{0.16}{4}}$=500=K��
���� ���⿼���˹���ɵ�Ӧ�á�ƽ��ͼ�������ƽ�ⳣ���ļ������ѧƽ���ƶ�ԭ�����ѶȽϴ�Ҫע�����Ũ������ƽ�ⳣ�����жϷ�Ӧ���еķ���


 ������ȫ��������ϵ�д�
������ȫ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ȫ�� | B�� | �ۢݢ� | C�� | �ܢݢ� | D�� | �٢ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | C2H6O | B�� | C3H8O | C�� | C4H10O | D�� | C5H12O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����Ȼ�̼���γɵķ�ɢϵ��������Һ | |
| B�� | ����ֱ����Na+С��������Ĥ | |
| C�� | �������ء����Ӳ�������ֽ | |
| D�� | �����Ȼ�̼���γɵķ�ɢϵ���ж����ЧӦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | M-3aNA-b | B�� | M-3aNA+b | C�� | [��M��NA��-3a-b] | D�� | [M�£�3a-b��NA] |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
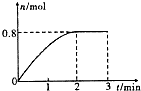 һ�������£���3molA��l mol B�����������ڹ̶��ݻ�Ϊ2L���ܱ������У��������·�Ӧ��3A��g��+B��g��?C��g��+2D��s��.2minĩ�÷�Ӧ�ﵽƽ�⣬����D�����ʵ�����ʱ��仯�����ͼ�������ж���ȷ���ǣ�������
һ�������£���3molA��l mol B�����������ڹ̶��ݻ�Ϊ2L���ܱ������У��������·�Ӧ��3A��g��+B��g��?C��g��+2D��s��.2minĩ�÷�Ӧ�ﵽƽ�⣬����D�����ʵ�����ʱ��仯�����ͼ�������ж���ȷ���ǣ�������| A�� | �����������ܶȲ��ٸı�ʱ���÷�Ӧ��һ���ﵽƽ��״̬ | |
| B�� | 2 min��ѹ��ʹ����Ӧ���ʼӿ죬�淴Ӧ���ʱ�����ƽ�������ƶ� | |
| C�� | ��Ӧ������A��B��ת����֮��Ϊ3��1 | |
| D�� | ��ʼ��ƽ�⣬��A��ʾ�÷�Ӧ�Ļ�ѧ��Ӧ����Ϊ0.3mol•L-1•min-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com