NaCl��һ�ֻ���ԭ�ϣ������Ʊ�һϵ�����ʣ���Ӧ���������ַ�Ӧ��������ȥ�������ǵ�ת����ϵ��ͼ��ʾ����ش��������⣺
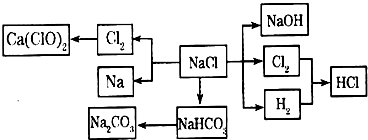
��1����ҵ�ϳ��õ������NaCl�ķ�����ȡ�����ƣ�NaCl�ۻ�ʱ�ƻ����Ӽ��Ĺ�������
�����仯
�����仯
��������仯����ѧ�仯������
��2��д����ҵ����ȡHCl�Ļ�ѧ����ʽ
��
��3��д����ҵ����ȡ�ռ�����ӷ���ʽ
��
��4������ƴ���Ĺ�������Ϊ��
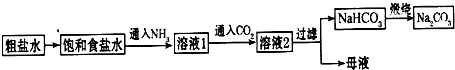
��NaHCO
3��ȡNa
2CO
3�Ļ�ѧ����ʽ
����������������ʳ��ˮ��ͨ��NH
3����ͨ��CO
2����Ŀ���ǣ�
��ΪNH3��������ˮ����Һ�������ԣ���ͨ��CO2ʹ֮�����ת��ΪHCO3-
��ΪNH3��������ˮ����Һ�������ԣ���ͨ��CO2ʹ֮�����ת��ΪHCO3-
��
��5���������һʵ��֤��Na
2CO
3��Һ���Ա�NaHCO
3��Һ�ļ���ǿ��
���� 0.1mol?L-1��������ʵ���Ũ�ȣ���������Һ������pH��Na2CO3��ҺpH����NaHCO3��Һ��˵��Na2CO3��Һ���Ա�NaHCO3��Һ�ļ���ǿ
���� 0.1mol?L-1��������ʵ���Ũ�ȣ���������Һ������pH��Na2CO3��ҺpH����NaHCO3��Һ��˵��Na2CO3��Һ���Ա�NaHCO3��Һ�ļ���ǿ
��





 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
![]() �����Է�ˮ��������ķ�������������ҵ��ˮ�м���������NaCl������ȣ�����Ϊ�缫���е�⣬�Ӷ�ʹ��Һ��pH��������ˮ������ת��Ϊ���ԣ�����һ��ʱ����Cr(OH)3��Fe(OH)3�������ɣ����˻��ճ�������ҵ��ˮ�и��ĺ����������ŷű���
�����Է�ˮ��������ķ�������������ҵ��ˮ�м���������NaCl������ȣ�����Ϊ�缫���е�⣬�Ӷ�ʹ��Һ��pH��������ˮ������ת��Ϊ���ԣ�����һ��ʱ����Cr(OH)3��Fe(OH)3�������ɣ����˻��ճ�������ҵ��ˮ�и��ĺ����������ŷű���![]() ��ΪCr3ʮ�����ӷ���ʽΪ________
��ΪCr3ʮ�����ӷ���ʽΪ________