
| VµÄȔֵ·¶Ī§ | m=f£ØV£© |

| 0.224L |
| 22.4L/mol |
| 1.86g-0.74g |
| 56g/mol |
| Vml |
| 22400ml/mol |
| mg |
| 100g/mol |
| V |
| 224 |
| (V-672)ml |
| 22400ml/mol |
| (1-m)g |
| 100g/mol |
| V-672 |
| 224 |
| VµÄȔֵ·¶Ī§ | m=f£ØV£© |
| 0”ÜV£¼224 | m=V/224 |
| 224ӆVӆ672 | m=1 |
| 672”ÜV”Ü896 | m=1-£ØV-672£©/224 |
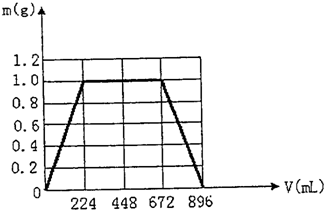 £¬¹Ź“š°øĪŖ£ŗ
£¬¹Ź“š°øĪŖ£ŗ £®
£®

 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø1£©µ±Éś³É³ĮµķµÄÖŹĮæøÕŗĆ×ī“óŹ±£¬ĻūŗÄCO2µÄĢå»ż224 mL£Ø±ź×¼×“æö£©”£ŌŚĻĀĶ¼ĖłŹ¾µÄ×ų±źĻµÖŠ£¬»³öÉś³É³ĮµķµÄÖŹĮæ£Øm£©ÓėĶØČėCO2µÄĢå»ż£ØV£©µÄ¹ŲĻµĶ¼”£Ķ¼ÖŠ£¬Éś³É³ĮµķµÄ×ī“óÖŹĮæĪŖ_____________g£¬³ĮµķøÕŗĆČ«²æČܽāŹ±ĻūŗÄCO2µÄĢå»żĪŖ_____________mL£¬»ģŗĻĪļÖŠKOHµÄÖŹĮæĪŖ_____________g”£
£Ø2£©ČōKOHŗĶCa(OH)2ŅŌČĪŗĪ±ČĄż»ģŗĻ£¬×ÜÖŹĮæČŌ±£³Ö1.3 g²»±ä£¬ŌņĻūŗÄCO2µÄ×ÜĢå»ż V µÄȔֵ·¶Ī§ĪŖ___________mL£¼V£¼___________mL”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
(10·Ö) ½«KOHŗĶCa£ØOH£©2»ģŗĻĪļ1.86gČ«²æČÜÓŚŅ»¶ØĮæĖ®ÖŠŠĪ³ÉĻ”ČÜŅŗ£¬ŌŁ»ŗ»ŗĶØČė×ćĮæµÄCO2ĘųĢ唣µ±Éś³É³ĮµķµÄÖŹĮæøÕŗĆ×ī“óŹ±£¬ĻūŗÄCO2µÄĢå»żĪŖ224mL£Ø±ź×¼×“æö£¬ŗöĀŌCO2ČÜÓŚĖ®Ēéæö”£ŅŌĻĀĒéæöĻąĶ¬”££©
£Ø1£©Éś³É³ĮµķµÄÖŹĮæøÕŗĆ×ī“óŹ±£¬·“Ó¦µÄ·½³ĢŹ½ĪŖ£ŗ __________________
£Ø2£©Ō»ģŗĻĪļÖŠCa£ØOH£©2µÄÖŹĮæĪŖ _________ g”£
£Ø3£©³ĮµķĒ”ŗĆČܽāŹ±£¬ĻūŗÄCO2µÄĢå»żĪŖ __________ mL
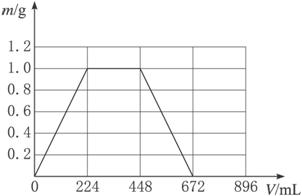
£Ø4£©Š“³öČÜŅŗÖŠÉś³É³ĮµķµÄÖŹĮæm£Øg£©ÓėĶØČėCO2µÄĢå»żV£ØmL£©Ö®¼äµÄŗÆŹż±ķ“ļŹ½”£
| VµÄȔֵ·¶Ī§ | m=f£ØV£© |
|
|
|
|
|
|
|
|
|
£Ø5£©ŌŚĶ¼Ź¾×ų±źĻµÉĻ£¬»³öÉś³É³ĮµķµÄÖŹĮæm£Øg£©ÓėĶØČėCO2µÄĢå»żV£ØmL£©µÄ¹ŲĻµĒśĻß”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ09”Ŗ10Äź³É¶¼ŹÆŹŅ֊ѧøßŅ»ĻĀĘŚĘŚÄ©æ¼ŹŌ»Æѧ¾ķ ĢāŠĶ£ŗ¼ĘĖćĢā
(10·Ö) ½«KOHŗĶCa£ØOH£©2»ģŗĻĪļ1.86gČ«²æČÜÓŚŅ»¶ØĮæĖ®ÖŠŠĪ³ÉĻ”ČÜŅŗ£¬ŌŁ»ŗ»ŗĶØČė×ćĮæµÄCO2ĘųĢ唣µ±Éś³É³ĮµķµÄÖŹĮæøÕŗĆ×ī“óŹ±£¬ĻūŗÄCO2µÄĢå»żĪŖ224mL£Ø±ź×¼×“æö£¬ŗöĀŌCO2ČÜÓŚĖ®Ēéæö”£ŅŌĻĀĒéæöĻąĶ¬”££©


£Ø1£©Éś³É³ĮµķµÄÖŹĮæøÕŗĆ×ī“óŹ±£¬·“Ó¦µÄ·½³ĢŹ½ĪŖ£ŗ __________________
£Ø2£©Ō»ģŗĻĪļÖŠCa£ØOH£©2µÄÖŹĮæĪŖ _________ g”£
£Ø3£©³ĮµķĒ”ŗĆČܽāŹ±£¬ĻūŗÄCO2µÄĢå»żĪŖ __________ mL
£Ø4£©Š“³öČÜŅŗÖŠÉś³É³ĮµķµÄÖŹĮæm£Øg£©ÓėĶØČėCO2µÄĢå»żV£ØmL£©Ö®¼äµÄŗÆŹż±ķ“ļŹ½”£
| VµÄȔֵ·¶Ī§ | m=f£ØV£© |
| | |
| | |
| | |




²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ09-10Äź³É¶¼ŹÆŹŅ֊ѧøßŅ»ĻĀĘŚĘŚÄ©æ¼ŹŌ»Æѧ¾ķ ĢāŠĶ£ŗ¼ĘĖćĢā
(10·Ö) ½«KOHŗĶCa£ØOH£©2»ģŗĻĪļ1.86gČ«²æČÜÓŚŅ»¶ØĮæĖ®ÖŠŠĪ³ÉĻ”ČÜŅŗ£¬ŌŁ»ŗ»ŗĶØČė×ćĮæµÄCO2ĘųĢ唣µ±Éś³É³ĮµķµÄÖŹĮæøÕŗĆ×ī“óŹ±£¬ĻūŗÄCO2µÄĢå»żĪŖ224mL£Ø±ź×¼×“æö£¬ŗöĀŌCO2ČÜÓŚĖ®Ēéæö”£ŅŌĻĀĒéæöĻąĶ¬”££©
£Ø1£©Éś³É³ĮµķµÄÖŹĮæøÕŗĆ×ī“óŹ±£¬·“Ó¦µÄ·½³ĢŹ½ĪŖ£ŗ __________________
£Ø2£©Ō»ģŗĻĪļÖŠCa£ØOH£©2µÄÖŹĮæĪŖ _________ g”£
£Ø3£©³ĮµķĒ”ŗĆČܽāŹ±£¬ĻūŗÄCO2µÄĢå»żĪŖ __________ mL
£Ø4£©Š“³öČÜŅŗÖŠÉś³É³ĮµķµÄÖŹĮæm£Øg£©ÓėĶØČėCO2µÄĢå»żV£ØmL£©Ö®¼äµÄŗÆŹż±ķ“ļŹ½”£
|
VµÄȔֵ·¶Ī§ |
m=f£ØV£© |
|
|
|
|
|
|
|
|
|
£Ø5£©ŌŚĶ¼Ź¾×ų±źĻµÉĻ£¬»³öÉś³É³ĮµķµÄÖŹĮæm£Øg£©ÓėĶØČėCO2µÄĢå»żV£ØmL£©µÄ¹ŲĻµĒśĻß”£


²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com