
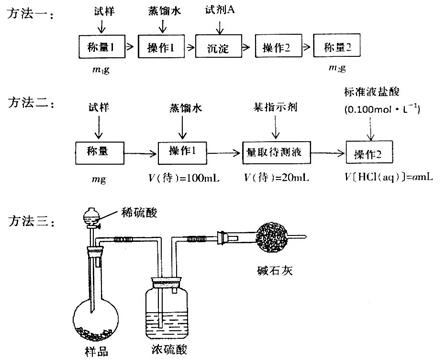
 £Ø»ņ
£Ø»ņ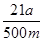 £©
£© Na2CO3£«H2O£«CO2”ü£ØĆææÕ1·Ö£©
Na2CO3£«H2O£«CO2”ü£ØĆææÕ1·Ö£©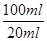 £½5a”Į10£4mol£¬ĘäÖŹĮ棽5a”Į10£4mol”Į84g/mol£½0.042ag£¬ĖłŅŌøĆѳʷ֊NaHCO3µÄÖŹĮæ·ÖŹżĪŖ
£½5a”Į10£4mol£¬ĘäÖŹĮ棽5a”Į10£4mol”Į84g/mol£½0.042ag£¬ĖłŅŌøĆѳʷ֊NaHCO3µÄÖŹĮæ·ÖŹżĪŖ ”£
”£ Na2CO3£«H2O£«CO2”ü”£
Na2CO3£«H2O£«CO2”ü”£


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®Ō×Ó°ė¾¶£ŗNa>Al |
| B£®Čō°ŃĀĮÄĘŗĻ½šĶ¶ČėŅ»¶ØĮæµÄĖ®ÖŠÖ»µĆµ½ĪŽÉ«ČÜŅŗ£¬Ōņn(Al)”Ün(Na) |
| C£®m g²»Ķ¬×é³ÉµÄĀĮÄĘŗĻ½šĶ¶Čė×ćĮæŃĪĖįÖŠ£¬Čō·Å³öH2Ō½¶ą£¬ŌņĀĮµÄÖŹĮæ·ÖŹżŌ½Š” |
| D£®ĀĮÄĘŗĻ½šĶ¶Čėµ½×ćĮæĀČ»ÆĶČÜŅŗÖŠ£¬æĻ¶ØÓŠĒāŃõ»ÆĶ³ĮµķÉś³É |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®¼ÓČėĖ®ÖŠ£¬²śÉśĪŽÉ«ĘųĢå |
| B£®¼ÓČė·ÓĢŖČÜŅŗÖŠ£¬ČÜŅŗĻȱäŗģŗóĶŹÉ« |
| C£®ŌŚøÉæÕĘųÖŠ¼ÓČȵ½400”ę£¬ÖŹĮæ¼õÉŁ |
| D£®ÓėSO2·“Ó¦ŗ󣬼ģ²āµ½ÓŠNa2SO4Éś³É |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗ¼ĘĖćĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
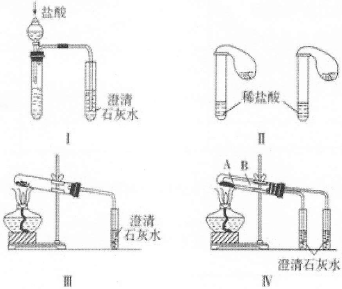
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
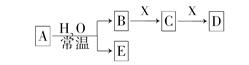
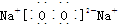
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
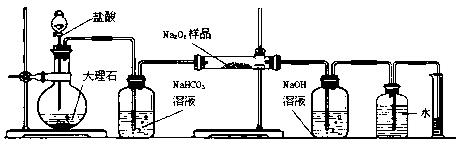
| A£® | B£® | C£® | D£®£ØE£©£ØF£© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®NaŗĶO2 | B£®NaOHŗĶCO2 | C£®NaHCO3ŗĶNaOH | D£®CŗĶO2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®Ö»ŗ¬Na2CO3 | B£®Ö»ŗ¬NaHCO3 |
| C£®NaOHŗĶNa2CO3µÄ»ģŗĻĪļ | D£®Na2CO3ŗĶNaHCO3µÄ»ģŗĻĪļ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com