
����Ŀ����Ԫ������Ȼ������Ҫ��������������Ҫ�ɷ�ΪAl2O3��������Fe2O3��FeO��SiO2���С���ҵ�����������Ʊ�����ij�ֻ�����Ĺ����������¡�
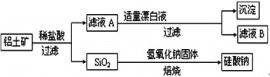
(1)����ҺA�м���Ư��Һ��������ҺB�����ԡ�
����ҺA�м���Ư��Һ��Ŀ���ǣ�________________________�������ӷ���ʽ��ʾ����
������ҺB�е���Ԫ���Գ�����ʽ��������ѡ�õ�����Լ�Ϊ������ţ�___________��
A������������Һ B��������Һ C����ˮ D��������̼
������Ӧ�����ӷ���ʽΪ____________
(2)������ҺB���Ƿ���Fe2+��ѡ����Լ�������Ϊ��_______________________________________
(3)������������������������������Һ�����Ӧ�����ӷ���ʽΪ________________________________________��
���𰸡�2Fe2++ClO-+5H2O=2Fe��OH��3��+C1-+4H+ C Al3++3NH3.H2O=Al(OH)3��+3NH4+ ���軯�ز�����ɫ���������Ը��������ɫ��ȥ SiO2+2OH- = SiO32-+ H2O��Al2O3 + 2 OH- = 2 AlO2- + H2O
��������
��������ϡ���ᷴӦ�����˺�õ�SiO2����ҺA����ҺA�к���Al3+��Fe3+��Fe2+,����ҺA�м���Ư��Һ��Ŀ��������������������ҺB�����ԣ�����������Ӿ���������,������������Ϊ����������Һ���γ���������������ȥ��������ҺB������,����Al3+�����ݴ˷������н��
��1����ҺA�м���Ư��Һ��Ŀ������������������������Ӿ���������������������Ϊ����������Һ���γ���������������ȥ�����ӷ���ʽΪ2Fe2++ClO-+5H2O=2Fe��OH��3��+C1-+4H+����Ԫ�ؼ�Al3+�Գ�����ʽ������Ҳ��������Al��OH��3����ѡ�õ�����Լ�Ϊ��ˮ�����Լӹ�����ˮ�����������ӷ���Ϊ��Al3++3NH3.H2O=Al(OH)3��+3NH4+��
�ʴ�Ϊ2Fe2++ClO-+5H2O=2Fe��OH��3��+C1-+4H+ ��C �� Al3++3NH3.H2O=Al(OH)3��+3NH4+
��2����ҺB���Ƿ���Fe2+�ķ���Ϊ�������軯�ز�����ɫ������������Ը��������ɫ��ȥ����û����ɫ��������ɫ����ȥ��˵����ҺB�в�����Fe2+��
�ʴ�Ϊ�� ���軯�ز�����ɫ���������Ը��������ɫ��ȥ��
��3����������Ҫ�ɷ�ΪAl2O3��������Fe2O3��FeO��SiO2������������������Һ����Al2O3��SiO2������Ӧ���ܽ�Al2O3��SiO2����Ӧ����ʽΪSiO2 +2OH- = SiO32- + H2O��Al2O3 + 2 OH- = 2 AlO2- + H2O��
�ʴ�Ϊ�� SiO2+2OH-= SiO32- + H2O�� Al2O3 + 2 OH- = 2 AlO2- + H2O��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��д�����л�����ĵ���ʽ��
��CO2:_______________����Na2O2:______________����N2:________________��
��2�����������ʣ��ٽ���ͭ ��NaOH ��I2 ��MgCl2 ��Na2O2 �����
�ش��������⣺
�ٲ����ڻ�ѧ������:______________________________________��
��ֻ���ڷǼ��Լ�����:_____________________________________��
��ֻ�������Ӽ�����:_______________________________________��
�� �ȴ������Ӽ��ִ��ڼ��Լ�����:___________________________��
�� �ȴ������Ӽ��ִ��ڷǼ��Լ�����:___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������H��һ���л��������м��塣ʵ�����ɷ��㻯����A�Ʊ�H��һ�ֺϳ�·�����£�

��֪����![]()
��![]()
�ش��������⣺
(1)A�й����ŵ�����Ϊ________����F����H�ķ�Ӧ����Ϊ________��
(2)E����F��ѧ����ʽΪ______________________��
(3)GΪ�ױ���ͬ���칹�壬G�Ľṹ��ʽΪ________ (д����ʽ)��
(4)���㻯����X��F��ͬ���칹�壬X���뱥��̼��������Һ��Ӧ�ų�CO2����˴Ź���������ʾ��4�ֲ�ͬ��ѧ�������⣬�������Ϊ6��2��1��1��д��2�ַ���Ҫ���X�Ľṹ��ʽ__________��
(5)д���ױ��ϳ�A�ĺϳ�·��(������ͼ��ʾ�����Լ���ѡ)__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������·�Ӧ����ʽ��
��SO3 + H2O��H2SO4�� ��2Na2O2+2H2O��4NaOH+ O2����
��2Na+2H2O��2NaOH+ H2���� ��SiO2+2NaOH��Na2SiO3+ H2O��
������Ҫ��������ѧ����ʽ��ĸ������Ӧ�ո��ڣ�
������Ӧ�в�����������ԭ��Ӧ����_________������ţ���ͬ����H2O������������_________������������ԭ��Ӧ�������е�H2O�Ȳ����������ֲ�����ԭ����______��
������֪����ʽF��KClO3��6HCl(Ũ)=KCl��3H2O��3Cl2����
(1)����˫���ŷ��������ת�Ƶķ������Ŀ___________________________________��
(2)��״���µ���33.6 L�������ų�ʱ��ת�Ƶ��ӵ���Ŀ��__________��
(3)������Ӧ����������ͻ�ԭ�����������Ϊ___________��
�����������£�K2Cr2O7��Һ��FeCl2��Һ��Ϻ�����Cr3+����д�����ӷ���ʽ __________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��һ����Ͷ�������Ĵ������ϵͳ���ŵ�ǰ����Ĥ�����ĵ����ΪNa2S2��NaBr3���ŵ��ֱ��ΪNa2S4��NaBr������������ȷ���ǣ� ��
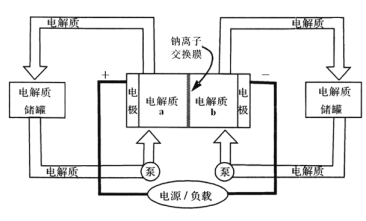
A. �ŵ�ʱ��������ӦΪ3NaBr��2e-=NaBr3+2Na+
B. ���ʱ��������ӦΪ2Na2S2��2e-=Na2S4+2Na+
C. �ŵ�ʱ��Na+�������ӽ���Ĥ����b������a��
D. �øõ�ص�ⱥ��ʳ��ˮ������2.24 L H2ʱ��b������17.40gNa2S4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͭ����������ʹ�õĺϽ�֮һ,��Ҫ��п��ͭ��ɡ��ش��������⣺
(1) ��̬пԭ�ӵĺ���۵����Ų�ʽΪ_______________,�������ڱ�__________��Ԫ�ء�����ռ������ܲ�ķ�����_______________ռ�ݸ��ܲ���ӵĵ���������ͼ��״Ϊ______________
(2)��һ������I1(Zn)________I1(Cu)(��������������С����)
(3)����ɫ ![]() ����ͭ��Һ�м����Թ����İ�ˮ,��Һ��Ϊ����ɫ[Cu(NH3)4] 2+��
����ͭ��Һ�м����Թ����İ�ˮ,��Һ��Ϊ����ɫ[Cu(NH3)4] 2+��
������������SO42-��Ϊ�ȵ��������__________(�����)��
A.H2SO4 B.CO32- C.PO43- D.CCl4
��H2O��������ԭ�ӵ��ӻ�����Ϊ______��NH3���ӵĿռ乹��Ϊ________��
�����еļ��ǣ�H2O_______ NH3(��������������С����)��
��ͨ������ʵ�������֪����Cu2+�����λ����H2O_________NH3 (��������������С����)��
������Ӧ��ǰ���İ����飨BH3��NH3�������黥Ϊ�ȵ����塣д��BH3��NH3�Ľṹʽ���ṹ��������λ������![]() ����ʾ��_______________________
����ʾ��_______________________
(4)����Cu�����е�ԭ�Ӷѻ���ʽ��ͼ��ʾ,���ֶѻ���ʽ��Ϊ_____________��

(5)��Cu������ܶ�Ϊ��g/cm3,![]() ��ʾ�����ӵ�������ֵ,��ʽ��ʾCu���������������Cuԭ��֮��ľ���________nm(���ػ���)
��ʾ�����ӵ�������ֵ,��ʽ��ʾCu���������������Cuԭ��֮��ľ���________nm(���ػ���)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ϩ����Ҫ�Ļ���ԭ�ϣ�����ϩΪԭ���ڲ�ͬ�����¿ɺϳ��������ʣ���������δ�������
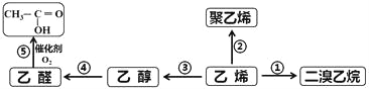
������Ҫ��д����
��1������ϩ�Ľṹ��ʽ��_____________����ȩ�Ľṹ��ʽ��_____________��
��2����Ӧ�ٵĻ�ѧ����ʽ��________________________________����Ӧ������___________��
��3����Ӧ�۵Ļ�ѧ����ʽ��________________________________����Ӧ������___________��
��4����Ӧ�ݵĻ�ѧ����ʽ��________________________________����Ӧ������___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ʵ����Ǹ��л�ѧ���õ�������������������йؼ�����
(1)0. 6 g H2�к�����ԭ�ӵ����ʵ���Ϊ_________ mol��
(2)��״���£�������ͬ��ԭ������CO��CO2�����֮��Ϊ____________��
(3)100mL��������Һ��n(Na+)=0.2mol��������c(SO42-)=_____________��
(4)6.72L(��״��)CO��һ������Fe2O3ǡ����ȫ��Ӧ������Fe������Ϊ____________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������X��Y��Z����Է���������ϵΪMr(X)<Mr(Y)��0.5Mr(Z)������˵����ȷ����
A.ԭ����Ŀ��ȵ��������壬����������Z
B.ͬ��ͬѹ�£�ͬ�������������壬�����ܶ���С����X
C.ͬ��ͬѹ�£��������������Ϊ6.72 L�������ǵ����ʵ���һ����Ϊ0.3 mol
D.ͬ���£������ͬ���������ֱ����2 g Y�����1 g Z���壬����ѹǿ��Ϊ2�U1
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com