
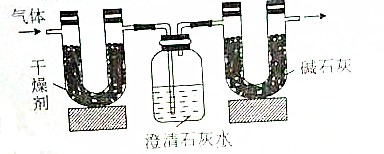
| ʵ��ǰ | ʵ��� | |
| �������+U�ιܣ������� | 51.10g | 52.45g |
| ������ʯ��ˮ+���ƿ�������� | 408.00g | 410.20 |
���� ��1����Ϊ��ȼ�Ϻ�C��H����Ԫ�أ���ȼ�ղ���ΪCO2��H2O���������֪U�ι����ӵ�����Ϊ����ˮ�����������ƿ���ӵ�����Ϊ���ɵ�CO2���������ɵ�����ΪCaCO3������̼ԭ���غ����CaCO3�����ʵ������ٸ���m=nM����CaCO3��������
��2������ˮ�ķ���ʽ��HԪ��������������HԪ��������
��3�����ݶ�����̼��̼Ԫ��������������CԪ��������
��4������C��Hԭ����Ŀ֮��ȷ�����ʽ�ͷ���ʽ��
��� �⣺��1����Ϊ��ȼ�Ϻ�C��H����Ԫ�أ���ȼ�ղ���ΪCO2��H2O���������֪��������ӵ�����Ϊ����ˮ������Ϊ52.45g-51.10g=1.35g�����ƿ���ӵ�����Ϊ���ɵ�CO2����Ϊ410.20-408.00g=2.2g��
���ƿ���ӵ�����Ϊ���ɵ�CO2������314.2g-312.0g=2.2g��CO2�����ʵ���=$\frac{2.2g}{44g/mol}$=0.05mol�����ƿ�����ɵ�����ΪCaCO3������Cԭ���غ��֪����CaCO3 0.05mol��������Ϊ0.05mol��100g/mol=5g��
�ʴ�Ϊ��1.35��5��
��2��ˮ�к�HԪ������Ϊ1.35g��$\frac{2}{18}$=0.15g��
�ʴ�Ϊ��0.15��
��3�����ɵ�0.05mol CO2����CԪ������Ϊ0.05mol��12g/mol=0.6g��
�ʴ�Ϊ��0.6��
��4������C��HԪ���غ㣬��ȼ����N��C����N��H��=0.05mol��$\frac{0.15g}{1g/mol}$=1��3���������ʽΪCH3�������ʽΪC2H6��
�ʴ�Ϊ��C2H6��
���� ���⿼����̽�����ʵ�����ɣ������ڿ����л����ȼ�շ���Ԫ���غ㷨ȷ���л������ʽ���Ѷ��еȣ�ע��Ի���֪ʶ���������գ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | 2H��g��+2X��g���T2HX��g����H3��0 | |
| B�� | ��H1=��H2+��H3 | |
| C�� | Cl��Br��I�ķǽ��������μ���������;�������յ�������Cl��Br��I��˳���������� | |
| D�� | ;��������HCl�ų�������������HBr�Ķ࣬˵��HCl��HBr�ȶ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ʵ��Ŀ�� | ��Ҫ���� | �Լ� |
| A | ����Br2��CCl4����� | ��Һ©�����ձ� | Br2��CCl4��������ˮ |
| B | ���������Ǻ����� | �Թܡ��ձ����ƾ��� | ��������Һ��������Һ��������Һ��ˮ |
| C | ʵ������ȡH2 | �Թܡ������ܵ���Ƥ�� | п����ϡHNO3 |
| D | �ⶨNaOH��ҺŨ�� | �ζ��ܡ���ƿ���ձ� | NaOH��Һ��0.1000mol•L-1���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  װ�ÿ��ռ�NO���� | |
| B�� |  װ�ÿ����ն��ఱ�����ܷ�ֹ���� | |
| C�� |  װ�ÿ�ʵ�ַ�Ӧ��2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2H2��+O2�� | |
| D�� |  װ�ÿ�֤���ǽ����ԣ�S��C��Si |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 һ��ѹǿ�£���ij�����ܱ������У�����H2��CO2������Ӧ��2CO2��g��+6H2��g��?CH3CH2OH��g��+3H2O��g��������ʼͶ�ϱȡ��¶���CO2��ת���ʵĹ�ϵ��ͼ��ʾ��
һ��ѹǿ�£���ij�����ܱ������У�����H2��CO2������Ӧ��2CO2��g��+6H2��g��?CH3CH2OH��g��+3H2O��g��������ʼͶ�ϱȡ��¶���CO2��ת���ʵĹ�ϵ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
 �����ʵ�������������Ӧ2Fe3++2I-?2Fe2++I2��Ƴ���ͼ��ʾ��ԭ��أ������ж���ȷ���ǣ�������
�����ʵ�������������Ӧ2Fe3++2I-?2Fe2++I2��Ƴ���ͼ��ʾ��ԭ��أ������ж���ȷ���ǣ�������| A�� | ��Ӧ��ʼʱ�����ӴӼ׳ص缫�����ҳص缫 | |
| B�� | ƽ��ʱ������ָ��Ϊ�㣬������Һ��ɫ��ͬ | |
| C�� | ƽ��ʱFe3+�����Ա�I2ǿ | |
| D�� | ƽ��ʱ��׳�������FeCl2������ҳص�ʯī�缫Ϊ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ��������������Һ��Ǩ�� | |
| B�� | �ŵ�����н���Ĥ�Ҳ���Һ��ɫ��dz | |
| C�� | �����ĵ缫��ӦʽΪFe3++3e-=Fe | |
| D�� | ��ת��2mole-������Ĥ�Ҳ���Һ��Լ����3 mol���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com