


 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ش��������⣺
��1�����̼������������Al3+�����ӷ���ʽΪ______________________________��
��2�������Ҽ����ռ������![]() �����ӷ���ʽΪ_____________________________��
�����ӷ���ʽΪ_____________________________��
��3����֤��ҺB��Fe3+����ȡ������Һ������_______________�����Լ����ƣ���
��4����ҺE��K�����ʵ���Ҫ�ɷ���_______________���ѧʽ����д�������ʵ�һ����;_______________��
��5����֪298 Kʱ��Mg(OH)2���ܶȻ�����Ksp=5.6��10-12��ȡ��������ҺB������һ�������ռ����ﵽ�����ܽ�ƽ�⣬���pH=13.00������¶��²�������Һ�е�c(Mg2+)=__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010������»���ѧ������ѧ�ڵ������¿���ѧ�� ���ͣ������
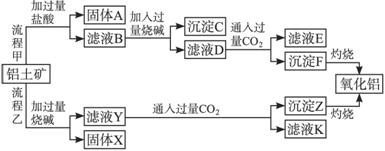
��������(��Ҫ�ɷ���Al2O3����SiO2��Fe2O3��MgO������)����ȡ���ֹ���Ʒ���������£�
��ش��������⣺
��1�����̼������������Al3+�ķ���ʽΪ ��
��2�������Ҽ����ռ������SiO32�������ӷ���ʽΪ ��
��3����֤��ҺB��Fe3+����ȡ������Һ������ �����Լ����ƣ���
��4����ҺE�����ʵ���Ҫ�ɷ��� ���ѧʽ����д�������ʵ�һ�־�����; ��
��5����֪298 Kʱ��Mg(OH)2���ݶȻ�����Ksp=5.6��10��12��ȡ��������ҺB������һ�������ռ�ﵽ������Һƽ�⣬���pH=13.00������¶��²�������Һ�е�c(Mg2+) = ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�������и߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��������±ʯ(K2SO4��MgSO4��2CaSO4��2H2O)���ڡ�������ˮ�д��������ܽ�ƽ�⣺
K2SO4��MgSO4��2CaSO4��2H2O(s) 2Ca2����2K����Mg2����4SO42-��2H2O��Ϊ�ܳ�����ü���Դ���ñ���Ca(OH)2��Һ�ܽ���±ʯ�Ʊ�����أ������������£�
2Ca2����2K����Mg2����4SO42-��2H2O��Ϊ�ܳ�����ü���Դ���ñ���Ca(OH)2��Һ�ܽ���±ʯ�Ʊ�����أ������������£�
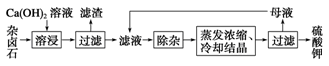
��1��������Ҫ�ɷ���________��CaSO4�Լ�δ����±ʯ��
��2���û�ѧƽ���ƶ�ԭ������Ca(OH)2��Һ���ܽ���±ʯ����K����ԭ��
��
��3�������ӡ������У��ȼ��� ��Һ��������Ȳ������ˣ��ټ��� ��Һ����ҺpH�����ԡ�
��4����ͬ�¶��£�K���Ľ���Ũ�����ܽ�ʱ��Ĺ�ϵ����ͼ����ͼ�ɵã������¶����ߣ�
�� ��
�� ��

���ܽ�����K����ƽ��Ũ������
��5�������Կ�����̼����Ϊ�ܽ��������ܽ������лᷢ����CaSO4(s)��CO32- CaCO3(s)��SO42-����֪298 Kʱ��Ksp(CaCO3)��2.80��10��9��Ksp(CaSO4)��4.90��10��5��������¶��¸÷�Ӧ��ƽ�ⳣ����K��
��
CaCO3(s)��SO42-����֪298 Kʱ��Ksp(CaCO3)��2.80��10��9��Ksp(CaSO4)��4.90��10��5��������¶��¸÷�Ӧ��ƽ�ⳣ����K��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ӱ�ʡʯ��ׯ�и������ڵڶ��ο��Ի�ѧ�Ծ��������棩 ���ͣ������
��6�֣���������(��Ҫ�ɷ���Al2O3����SiO2��Fe2O3��MgO������)����ȡ���ֹ���Ʒ���������£�
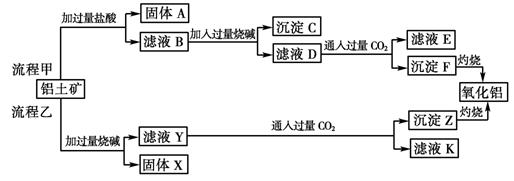
��ش��������⣺
(1)���̼������������Al3�������ӷ���ʽΪ ��
(2)�����Ҽ����ռ������SiO32�������ӷ���ʽΪ ��
(3)��֤��ҺB��Fe3������ȡ������Һ������ (���Լ�����)��
(4)��ҺE��K�����ʵ���Ҫ�ɷ��� (�ѧʽ)��д������Һ��һ����;
��
(5)��֪298 Kʱ��Mg(OH)2���ܶȻ�����Ksp��5.6��10��12��ȡ��������ҺB������һ�������ռ�ﵽ�����ܽ�ƽ�⣬���pH��13.00������¶��²�������Һ�е�c(Mg2��)�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ����10�½β��Ի�ѧ�Ծ��������棩 ���ͣ������
��10�֣���������(��Ҫ�ɷ���Al2O3����SiO2��Fe2O3��MgO������)����ȡ�����������ֹ����������£�

��ش��������⣺
��1�����̼�ͨ�����CO2�����ɳ���F�����ӷ�Ӧ����ʽΪ______________________________________��
(2�����Ҽ����ռ��ܽ�SiO2�Ļ�ѧ��Ӧ����ʽ____________________��
(3)��֤��ҺB��Fe3������ȡ������Һ������________(���Լ�����)��
(4)��ҺE��K�����ʵ���Ҫ�ɷ���________(�ѧʽ)��д�������ʵ�һ����;____________________��
(5)��֪298 Kʱ��Mg(OH)2���ܶȻ�����Ksp��5.6��10��12��ȡ��������ҺB������һ�������ռ����ﵽ�����ܽ�ƽ�⣬���pH��12������¶��²�������Һ�е�c(Mg2��)��________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com