
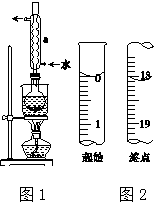
 Na2S2O3����Ҫ�Ļ���ԭ�ϣ�������ˮ�������Ի���Ի������ȶ���
Na2S2O3����Ҫ�Ļ���ԭ�ϣ�������ˮ�������Ի���Ի������ȶ������� ��1�����������ˮ�������Ҵ����Ҵ�ʪ�����ʹ������ڷ�ɢ����Һ�У�
��2������ͼʾװ������������д�������ƣ�Ȼ������������ܹ����������������ý��н��
��3������S2O32?���л�ԭ�ԣ��ױ�������������������ӿ�֪����Ϊ�����ƣ����ݼ�����������ӵķ����������������ƣ�
��4��S2O32?������������Һ���ܹ�����������ԭ��Ӧ�������ʣ��ݴ�д����Ӧ�����ӷ���ʽ��
��5�����ݵζ�ǰ��ҺΪ��ɫ���ζ������ⵥ��ʹ���۱������жϴﵽ�յ�ʱ��Һ��ɫ�仯��
��6������ͼʾ�ĵζ�����Һ��������������ն�����Ȼ���������ĵ�ı���Һ��������ݷ�Ӧ2S2O32-+I2�TS4O62-+2I-��֪��n��S2O32-��=2n��I2����Ȼ��������еⵥ�ʵ����ʵ��������Na2S2O3•5H2O��������Ʒ�Ĵ��ȣ�
��7�����������Ϣ��Na2S2O3��ԭ�Խ�ǿ������Һ���ױ�Cl2������SO42-�������ϼ��������д����Ӧ�����ӷ���ʽ��
��� �⣺��1�����������ˮ�����Ҵ�����������ڷ�Ӧǰ���Ҵ�ʪ����ʹ������ڷ�ɢ����Һ�У�
�ʴ�Ϊ��ʹ������ڷ�ɢ����Һ�У�
��2����������ͼʾװ��ͼ��֪������aΪ�����ܣ���ʵ���������ܾ����������������ã�
�ʴ�Ϊ�������ܣ�����������
��3��S2O32?���л�ԭ�ԣ��ܹ���������������������ӣ����Կ��ܴ��ڵ������������ƣ����������Ƶķ���Ϊ��ȡ������Ʒ���ڹ���ϡ���ᣬ���ˣ�����Һ�м�BaCl2��Һ�����а�ɫ���������Ʒ�к���Na2SO4��
�ʴ�Ϊ��Na2SO4�� ȡ������Ʒ���ڹ���ϡ���ᣬ���ˣ�����Һ�м�BaCl2��Һ�����а�ɫ���������Ʒ�к���Na2SO4��
��4��S2O32?�������ӷ���������ԭ��Ӧ���ɵ���ɫ���ʣ���Ӧ�����ӷ���ʽΪ��S2O32?+2H+=S��+SO2��+H2O��
�ʴ�Ϊ��S2O32?+2H+=S��+SO2��+H2O��
��5���ζ������ⵥ��ʹ���۱��������Եζ��յ�ʱ��Һ��ɫ�仯Ϊ������ɫ��Ϊ��ɫ��
�ʴ�Ϊ������ɫ��Ϊ��ɫ��
��6������ͼʾ�ĵζ�����Һ���֪���ζ����г�ʼ����Ϊ0���ζ��յ�Һ�����Ϊ18.10mL���������ĵ�ı���Һ���Ϊ18.10mL��
���ݷ�Ӧ2S2O32-+I2�TS4O62-+2I-��֪��n��S2O32-��=2n��I2��������W g��Ʒ�к���Na2S2O3•5H2O����Ϊ��0.1000 mol•L-1��18.10��10-3L��2��M=3.620��10-3Mg�����Ʒ�Ĵ���Ϊ��$\frac{3.620��10{\;}^{-3}Mg}{Wg}$��100%=$\frac{3.620��10{\;}^{-3}M}{W}$��100%��
�ʴ�Ϊ��18.10��$\frac{3.620��10{\;}^{-3}M}{W}$��100%��
��7��Na2S2O3��ԭ�Խ�ǿ������Һ���ױ�Cl2������SO42-�����ݻ��ϼ����������ƽ������ӷ���ʽΪ��S2O32?+4Cl2+5H2O=2SO42?+8Cl?+10H+��
�ʴ�Ϊ��S2O32?+4Cl2+5H2O=2SO42?+8Cl?+10H+��
���� ���⿼���˻�ѧʵ������������������������Ĺ��졢���ӵļ��鷽�����к͵ζ����ڼ����㡢���ӷ���ʽ����д��֪ʶ����Ŀ�ѶȽϴ������漰�������ϴ�֪ʶ��϶࣬��ֿ�����ѧ������ѧ֪ʶ�����������


 ���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | һ�������·�ӦN2+3H2$?_{����}^{����}$2NH3���ﵽƽ��ʱ��3v����H2��=2v����NH3�� | |
| B�� | 10mLŨ��Ϊ1mol/L�������������Zn�۷�Ӧ��������������CH3COONa��Һ�����ܽ��ͷ�Ӧ���ʣ��ֲ�Ӱ��H2������ | |
| C�� | ��pH=a+1�İ�ˮϡ��ΪpH=a�Ĺ����У�c��OH-��/c��NH3•H2O����С | |
| D�� | �����£���Ũ��Ϊ0.1mol/L��CH3COONa��Һ�м���������Ũ�ȵ�CH3COOH�������Һ��pH=7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ������; | ���� | |
| A | ʳ����ˮ���е�ˮ�� | ˮ����CaCO3���ڴ��ᣬ����H2CO3��CH3COOH |
| B | ����ʱ��һ��ƺʹ� | �������������ɣ�ʹ��ζ��ɿ� |
| C | NaClO��Һ������ϴ��Һ | NaClO����ɱ������������ |
| D | Al2O3������ҽҩ�е�θ���кͼ� | Al2O3����θ�ᷴӦ��ʹθҺ��Ƚ��� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.01mol/L NH4Al��SO4��2��Һ��0.01mol•L-1Ba��OH��2��Һ��������NH4++Al3++2SO42-+2Ba2++4OH-=2BaSO4��+Al��OH��3��+NH3•H2O | |
| B�� | �ö��Ե缫���CuCl2��Һ��2Cu2++2H2O$\frac{\underline{\;���\;}}{\;}$2Cu+O2��+4H+ | |
| C�� | ����״���µ�11.2L����ͨ��200mL2mol•L-1��FeBr2��Һ�У����ӷ�Ӧ����ʽΪ��4Fe2++6Br-+5Cl2=4Fe3++3Br2+10Cl- | |
| D�� | �����еμ�����Ũ���Fe+3NO3-+6H+=Fe3++3NO2��+3H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʹ������ƿǰ����������ƿ�Ƿ�©ˮ | |
| B�� | ����ƿ������ˮϴ�������ô�����Һ��ϴ | |
| C�� | �ƺõĹ�����������ֽ��С�ĵ���������ƿ�� | |
| D�� | ҡ�Ⱥ��ְ�Һ���½����ټ�ˮ���̶��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com