
 ����ͼ��ʾװ�ý���ʵ�飬��A��μ���B�У�
����ͼ��ʾװ�ý���ʵ�飬��A��μ���B�У�| �� |
| �� |
| �� |
| �� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 (4��)��(1)�¶ȼƳ�����������ƻ�ѧʵ����¶ȡ�
(4��)��(1)�¶ȼƳ�����������ƻ�ѧʵ����¶ȡ�
��˵������ʵ�����¶ȼƵ�ˮ����������λ�ã�ʵ��������ϩ____________________���������л����ķ���_______________________________��
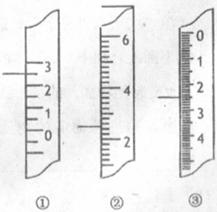
(2)��ͼ�ֱ����¶ȼơ���Ͳ���ζ��ܵ�һ���֣�
��������(���ʵ�߱�עλ��)��˵����ȷ����_______ ��
A��������Ͳ������Ϊ2.5mL
B��������Ͳ������Ϊ2.5 mL
 C�����ǵζ��ܣ�����Ϊ3.5 mL
C�����ǵζ��ܣ�����Ϊ3.5 mL
D�������¶ȼƣ�����Ϊ2.
(3)ijͬѧ����ͼ��ʾװ�ã������巢��װ���в������Ȼ���ֱ��ͨ��ˮ�������������ᣬ���������ˮ�ĵ������ڲ���������(��ѡ�������Լ�)��ǰ���½���ͼװ���ԼӸĽ������ɰ�ȫ�����Թ�����ˮ�����Ȼ��⣬�Ľ��ķ�����______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ִ���ҵ��ú̿�����ȿ������ȼ�ϵ������ʡ�����CO��SO2�ȵ��ŷţ��ֿ����Ƴ����������Դ������ˮú���Ĺ㷺��;��
��1����֪����2C(s)+O2(g)=2CO(g) ��H1=��221.0kJ.mol-1��
��2H2(g)+O2(g)=2H2O��g����H2=��483.6kJ.mol-1
��ӦC(s)+ H2O��g��= CO(g) + H2(g)����H=____ ��
��2����ҵ����һ����CO2 �������״�ȼ�ϵķ����� CO2(g)��3H2(g)![]() CH3OH(g)��H2O(g) ��H����49.0 kJ��mol��1
CH3OH(g)��H2O(g) ��H����49.0 kJ��mol��1
 �ֽ�6.0molCO2��8.0molH2����2L���ܱ������У����H2�����ʵ�����n����ʱ��仯����ͼ��ʾ��ʵ�ߣ���
�ֽ�6.0molCO2��8.0molH2����2L���ܱ������У����H2�����ʵ�����n����ʱ��仯����ͼ��ʾ��ʵ�ߣ���
������ʱ���ƽ����Ӧ����������______������ţ���
A��0��1min B��1��3min
C��3��8min D��8��11min
�������ǣ���(H2)=_________ ��
�� ƽ��ʱ������ת���� ����������
��Ӧ��ƽ�ⳣ��K= ��
�۽��ı�ijһʵ�������ٽ�������ʵ�飬���H2�����ʵ�����ʱ��仯��ͼ��������ʾ������I��Ӧ��ʵ�������ı���___________������II��Ӧ��ʵ�������ı���_____________��
��3��ij�о�����װ��CH3OH��O2ȼ�ϵ�ص�
����ԭ����ͼ��ʾ��

�ٸõ�ع���ʱ��b��ͨ�������Ϊ ��
�ڸõ�������ĵ缫��ӦʽΪ�� ��
 ���Դ˵������Դ����ʵ������ģ������Ʒ����
���Դ˵������Դ����ʵ������ģ������Ʒ����
���ۻ���������װ����ͼ��ʾ���Ĺ����У�������Һ
����Dz������ݲ�������ԭ�������
������ص����ӷ���ʽ��ʾ����
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com