
����Ŀ��������[ (CH3COO)2Ni]��һ����Ҫ�Ļ���ԭ�ϡ�һ���Ժ�������(��NiS��Al2O3��FeO��CaO��SiO2) Ϊԭ�ϣ���ȡ�������Ĺ�������ͼ����:
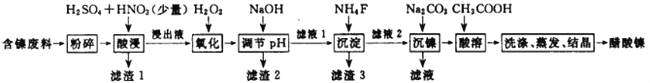
��������������������pH��������ʵ��ܽ������±���
��1��������ĺ����������ʱҪ���Ͻ��裬����ͽ����Ŀ����______________________________��
��2������pH�����У���ҺpH�ĵ��ڷ�Χ��_______________________________��
��3������1������3��Ҫ�ɷֵĻ�ѧʽ�ֱ���____________��_____________��
��4�����������м���H2O2������Ӧ�����ӷ���ʽ________________________________��
��5����������У�lmol NiSʧȥ6NA�����ӣ�ͬʱ����������ɫ�ж����塣д���÷�Ӧ�Ļ�ѧ����ʽΪ____________________________________��
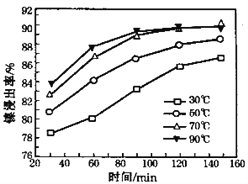
��6�����������������䣬�ڲ�ͬ�¶��¶Ժ��� ���Ͻ������������������ʱ��仯����ͼ�� ���������¶���ʱ��ֱ�Ϊ________�桢 _______min��
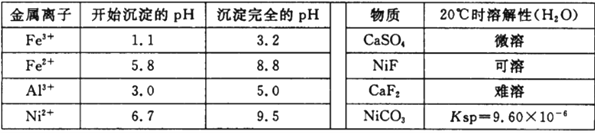
���𰸡� ����Ӵ��������߽������ʺͲ��ʣ�������ʣ� 5.0��pH��6.7 SiO2��CaSO4 CaF2 2Fe2++H2O2+2H+= 2Fe3++2H2O NiS+H2SO4+2HNO3== NiSO4+SO2��+2NO��+2H2O 70 120
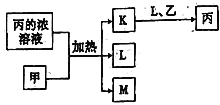
����������������(�� NiS��Al2O3��FeO��CaO��SiO2)���飬�������������������ˣ�����1Ϊ�������������ƣ�����Һ����Ni2+��Fe2+��Al3+��Ca2+����H2O2��������������ΪFe3+��Ȼ���NaOH����pH��ʹAl3+��Fe3+ת��Ϊ������ͬʱNi2+����ת��Ϊ���������Ե���pH�ķ�Χ5.0��pH��6.7�����ˣ�����2Ϊ����������������������Һ�к���Ni2+��Ca2+���ټӷ���泥�����CaF2���������ˣ�����3ΪCaF2����Һ�м�̼��������NiCO3���������ˣ������мӴ����ܽ⣬����(CH3COO)2Ni��Һ��Ȼ������Ũ������ȴ�ᾧ�õ�(CH3COO)2Ni���塣
(1)������ĺ����������ʱҪ���Ͻ��裬����ͽ����������Ӵ��������߽������ʺͲ��ʣ��ʴ�Ϊ������Ӵ��������߽������ʺͲ��ʣ�
(2)����pHʹAl3+��Fe3+ת��Ϊ������ͬʱNi2+����ת��Ϊ���������ݱ��е����ݿ�֪������pH�ķ�ΧΪ5.0��pH��6.7���ʴ�Ϊ��5.0��pH��6.7��
(3)�����̷�����֪������1Ϊ�������������ƣ�����3ΪCaF2���ʴ�Ϊ��SiO2��CaSO4��CaF2��
(4)��Һ�е��������Ӳ�����ת��Ϊ��������H2O2��������������ΪFe3+����Ӧ�����ӷ���ʽΪ��2Fe2++H2O2+2H+�T2Fe3++2H2O���ʴ�Ϊ��2Fe2++H2O2+2H+�T2Fe3++2H2O��
(5)��������У�1mol NiS����������ʧȥ6NA�����ӣ�ͬʱ����������ɫ�ж����壬������NO��SO2���䷴Ӧ�Ļ�ѧ����ʽΪ��NiS+H2SO4+2HNO3�TNiSO4+SO2��+2NO��+2H2O���ʴ�Ϊ��NiS+H2SO4+2HNO3�TNiSO4+SO2��+2NO��+2H2O��
(6)��ͼ���֪��70����120minʱ�����������ߣ��ʴ�Ϊ��70��120��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Ƭ�����������ð֢״����ṹ��ʽ��ͼ�������й�˵���������

A. ����ҵķ���ʽΪC13H18O2 B. ������뱽������ͬϵ��
C. 1mol������������3mol���������ӳɷ�Ӧ D. ������ڱ����Ϸ���ȡ����Ӧ����һ�ȴ�����4��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͬ��ͬѹ�£�����������ɱ���ܱ������зֱ������������O2��O3���壬����˵����ȷ����
A. ����������ܶ�֮����3��2 B. �������������֮��Ϊ2��3
C. ��������ķ�����Ŀ��� D. ����������ʵ���֮��Ϊ3��2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������Ӽ���ķ�����ȷ����
A. ij��Һ![]() �а�ɫ������˵��ԭ��Һ����Cl��
�а�ɫ������˵��ԭ��Һ����Cl��
B. ij��Һ![]() �а�ɫ������˵��ԭ��Һ����SO42��
�а�ɫ������˵��ԭ��Һ����SO42��
C. ij��Һ![]() ����ɫ������˵��ԭ��Һ����Cu2+
����ɫ������˵��ԭ��Һ����Cu2+
D. ij��Һ![]() ������ɫ���壬˵��ԭ��Һ����CO32��
������ɫ���壬˵��ԭ��Һ����CO32��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ï��������ȼ�ϵĽ������̼����������ȡ�ʵ�����Ʊ���ï��װ��ʾ��ͼ������ͼ����ï���۵���173�棬��100��ʱ��ʼ�������е���249�棩��ʵ�鲽��Ϊ��
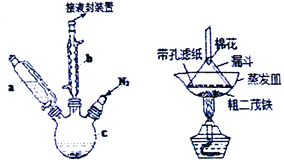
����������ƿ�м���25g��ĩ״��KOH����������a�м���60mL��ˮ���ѵ���ƿ�У���ֽ��裬ͬʱͨ����Լ10min��
���ٴ�����a����5.5mL������Ļ����ϩ���ܶ�0.95g/cm3�������裻
�۽�6.5g��ˮFeCl2��(CH3)2SO���������������ܼ�����ɵ���Һ25mLװ������a�У�������������c�У�45min���꣬��������45min��
���ٴ�����a����25mL��ˮ���ѽ��裻
�ݽ�c�е�Һ��ת������d�����������ᡢˮ��ϴ�����Σ���Һ�óȻ�ɫ��Һ��
�������Ȼ�ɫ��Һ���ö�ï���ֲ�Ʒ��
�ش��������⣺
��1������b��������______________��������________________________��
��2���������ͨ�뵪����Ŀ����__________________________________��
��3������c�������ݻ�ӦΪ_________����100mL����250mL����500mL������aʹ��ǰӦ���еIJ�����_______________________________________��
��4��KOH��FeCl2��C5H6��Ӧ���ɶ�ï��[Fe(C5H5)2]��KCl�Ļ�ѧ����ʽΪ_________________________��������Ƕ�ï���ֲ�Ʒ���ᴿ���ù�������ͼ�н��У����������Ϊ ____________________________��
��5��Ϊ��ȷ֤�õ����Ƕ�ï��������Ҫ���е�һ���ʵ����_________________________��
��6�����յõ������Ķ�ï��3.7g�����ʵ��IJ���Ϊ __________________��������λ��Ч���֣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�������ͼ����ᰬ˾���(F)��һ�ֺϳ�·�����£�


��֪��
�ش��������⣺
��1��A�Ļ�ѧ������____________��F�к��������ŵ�������___________________��
��2����B����C�ķ�Ӧ����Ϊ____________����D����E�ķ�Ӧ����Ϊ____________��
��3��B�Ľṹ��ʽΪ____________��
��4����C����D�Ļ�ѧ����ʽΪ____________��
��5����ȡ�������廯����X��E��ͬ���칹�壬1molX��������NaHCO3��Ӧ������44.8L����״����CO2����˴Ź���������ʾ��5�ֲ�ͬ��ѧ�������⣬��������֮��Ϊ9:2:2:2:1��д��2�ַ���Ҫ���X�Ľṹ��ʽ��________________________________________________��
��6��д���Ա��״��ͱ�����Ϊԭ���Ʊ� �ĺϳ�·��_____________���������Լ���ѡ����
�ĺϳ�·��_____________���������Լ���ѡ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������²����![]() ��(�����Լ��ͷ�Ӧ��������ȥ)
��(�����Լ��ͷ�Ӧ��������ȥ)

��ش��������⣺
��1��A������Ϊ________________��
��2���ֱ�д�� B��D �Ľṹ��ʽ��B_________________��D__________________��
��3����Ӧ��������������ȥ��Ӧ����__________��
��4��D��Br2��1��2��Ӧ�IJ���Ľṹ��ʽΪ________________��
��5����д�� C�D��D ��Ӧ�Ļ�ѧ����ʽ___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������Ԫ��W��X��Y��Z��ԭ�������������ӡ�K��L��M��������ЩԪ����ɵĶ�Ԫ������ס��ҷֱ���Ԫ��X��Y�ĵ��ʣ����dz����Ĺ��壬���dz��������塣K����ɫ���壬����Ҫ�Ĵ�����Ⱦ��֮һ��0.05mol/L����Һ��pHΪ1,�������ʵ�ת����ϵ��ͼ��ʾ������˵����ȷ����
A. Ԫ�صķǽ�����:Z>Y>X
B. ��Ҳ����W��Y��ɵ�ij�ֻ�������Kֱ�ӷ�Ӧ�Ƶ�
C. ԭ�Ӱ뾶:W<X<Y
D. K��L��M�зе���ߵ���M
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com