
 H2 ��g��+
H2 ��g��+ X2 ��g��?HX ��g����ƽ�ⳣ��Ϊ10������1.0mol��HX ��g��ͨ�����Ϊ 1.0L���ܱ������У��ڸ��¶�ʱ HX ��g�������ֽ���ԼΪ ��
X2 ��g��?HX ��g����ƽ�ⳣ��Ϊ10������1.0mol��HX ��g��ͨ�����Ϊ 1.0L���ܱ������У��ڸ��¶�ʱ HX ��g�������ֽ���ԼΪ ��  H2 ��g��+
H2 ��g��+ X2 ��g��?HX ��g��
X2 ��g��?HX ��g��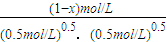 =10��x=
=10��x=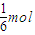 ��������ֽ���=
��������ֽ���= =17%��
=17%��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ�Ž�ͬ����ѧ�߶���ѧ���¿���ѧ�Ծ����������� ���ͣ������
��10�֣���1�����������ʵ���Ũ����ͬ��a HF��b NH3��H2O��c H2S��Һ�����볣���ֱ�Ϊ7.2��10-4��5.6��10-10��K1=9.1��10-8,K2=1.1��10-12��
�ٵ���ʵ�ǿ��˳��Ϊ��___________��(��a��b��c)����������Ũ����С���ǣߣߣ�(��a��b��c)��
��2��1L1mol/LH2SO4��Һ��2L1mol/LNaOH��Һ��ȫ��Ӧ���ų�114.6kJ���������ɴ���֪H2SO4��NaOH��Ӧ���к��ȵ��Ȼ�ѧ����ʽΪ___________________________��
(3)��1L1mol/L��NaOH��Һ�м����������ʣ���ŨH2SO4����ϡ�����ϡ���ᣬǡ����ȫ��Ӧ����ЧӦ��H1����H2����H3�Ĵ�С��ϵΪ ��
(4)��֪���з�Ӧ�ķ�Ӧ��:
��CH3COOH(l) + 2O2 (g)= 2CO2 (g)+ 2H2O(l) ��H=-870.3kJ�Mmol
��C(S) + O2 (g)= CO2 (g) ��H=-393.5kJ�Mmol
��2C(S) + 2H2 (g) + O2 (g)= CH3COOH(l) ��H=-488.3kJ�Mmol
��д��H2ȼ���ȵ��Ȼ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015������ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
����a��e����ѧ��ѧʵ���г����ļ��ֶ���������
(a)��Ͳ (b)����ƿ (c)�ζ��� (d)������ƽ (e)�¶ȼ�
��1���ޡ�0���̶ȵ��� (��д���)��
��2�����в����������� (����ĸ)
A����25mL��ʽ�ζ�����ȡ20.00mLNaHCO3
B����������ƽȷ����10.20��̼���ƹ���
C����100mL��Ͳ��ȡ3.2mLŨ����
D������1 mol��L�C1������������Һ475mLѡ��500mL����ƿ

��3��ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һ�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ������ͼ��ʾ��������������Һ�����Ϊ mL��
��4��ͼ�ױ�ʾ10 mL��Ͳ��Һ���λ�ã�A��B��B��C�̶ȼ����1 mL������̶�AΪ4����Ͳ��Һ������Ϊ mL��ͼ�ұ�ʾ25 mL��ʽ�ζ�����ij��������D��E ֮��Ŀ̶Ȳ�Ϊ1 mL������̶�DΪ4�������ʽ�ζ�����Һ������Ķ���Ϊ mL��

ͼ�� ͼ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�콭��ʡ�߶���ѧ���¿���ѧ�Ծ��������棩 ���ͣ������
��10�֣���1�����������ʵ���Ũ����ͬ��a HF��b NH3��H2O��c H2S��Һ�����볣���ֱ�Ϊ7.2��10-4��5.6��10-10��K1=9.1��10-8,K2=1.1��10-12��
�ٵ���ʵ�ǿ��˳��Ϊ��___________��(��a��b��c)����������Ũ����С���ǣߣߣ�(��a��b��c)��
��2��1L1mol/LH2SO4��Һ��2L1mol/LNaOH��Һ��ȫ��Ӧ���ų�114.6kJ���������ɴ���֪H2SO4��NaOH��Ӧ���к��ȵ��Ȼ�ѧ����ʽΪ___________________________��
(3)��1L1mol/L��NaOH��Һ�м����������ʣ���ŨH2SO4����ϡ�����ϡ���ᣬǡ����ȫ��Ӧ����ЧӦ��H1����H2����H3�Ĵ�С��ϵΪ ��
(4)��֪���з�Ӧ�ķ�Ӧ��:
��CH3COOH(l) + 2O2 (g)= 2CO2 (g)+ 2H2O(l) ��H=-870.3kJ�Mmol
��C(S) + O2 (g)= CO2 (g) ��H=-393.5kJ�Mmol
��2C(S) + 2H2 (g) + O2 (g)= CH3COOH(l) ��H=-488.3kJ�Mmol
��д��H2ȼ���ȵ��Ȼ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com