
��6�֣�����ȼ������Һ̬ˮ���Ȼ�ѧ����ʽ�ǣ�2H2(g)+O2(g) ===2H2O(l)����H=��572kJ/mol ��ش��������⣺
��1�������������ܺ���������������ڡ�����С�ڡ����ڡ�����Ӧ�������ܺ͡�
��2����2 mol������ȫȼ������ˮ��������ų������� 572 kJ �����������������������
��3����������������ɴ���ʹ����һ������װ�ã��乹����ͼ��ʾ��a��b�����缫���ɶ��̼����ɡ����ǽ������� ������ת��Ϊ���������ܵ�װ�á�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ȼ������Һ̬ˮ���Ȼ�ѧ����ʽ��
����ȼ������Һ̬ˮ���Ȼ�ѧ����ʽ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ȼ������Һ̬ˮ���Ȼ�ѧ����ʽ��2H2��g��+O2��g���T2H2O��l����H=-572kJ/mol
����ȼ������Һ̬ˮ���Ȼ�ѧ����ʽ��2H2��g��+O2��g���T2H2O��l����H=-572kJ/mol�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
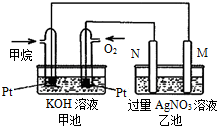 ��Ȼ������Ҫ�ɷּ���ȼ�����ɶ�����̼��Һ̬ˮ���Ȼ�ѧ����ʽ���£���ش��������⣺CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6kJ/mol��
��Ȼ������Ҫ�ɷּ���ȼ�����ɶ�����̼��Һ̬ˮ���Ȼ�ѧ����ʽ���£���ش��������⣺CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6kJ/mol���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com