
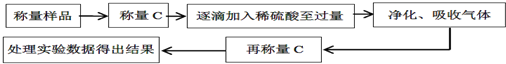
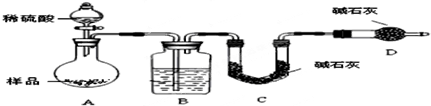



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
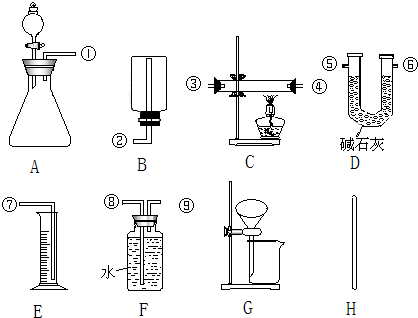
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��һ�� | �ڶ��� | ������ | |
| ��Ʒ������/g | 7.540 | 15.08 | 35.00 |
| ������������/L | 0.6720 | 1.344 | 2.688 |
| �������/g | 0.8000 | 1.600 | 3.200 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ζ�ǰ�ζ����������ݣ��ζ���������� |
| B����ʽ�ζ�����ȡNaOH��Һʱ��δ������ϴ���� |
| C���ζ�ʱ�ﵽ�ζ��յ�ʱ���Ӷ��� |
| D����ƿʢװNaOH����Һǰ������ˮϴ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������Һ�������ǣ������� |
| B���Ҵ������ᣩ����KOH��Һ����Һ |
| C���״���Һ�����ᣩ����NaOH��Һ������ |
| D������Һ�����ͣ�����ʳ�ν��衢���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
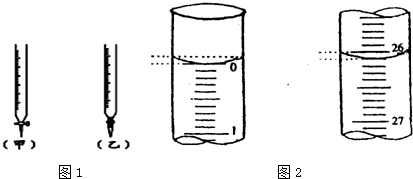
| ��ƿ����Һ | �ζ�������Һ | ָʾ�� | �ζ��� | |
| A | �� | �� | ʯ�� | ���ң� |
| B | �� | �� | ���� | ���ף� |
| C | �� | �� | ��̪ | ���ף� |
| D | �� | �� | ʯ�� | ���ң� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| A��X����������̼ԭ�ӿ��ܴ���ͬһ��ƽ���� |
| B��1 mol X��Ũ��ˮ��Ӧʱ�������2mol Br2 |
| C��X���Է����ӳɡ��Ӿۡ���������ԭ����ȥ�ȷ�Ӧ |
| D��1��X������H2��ȫ��Ӧ�IJ����к���3������̼ԭ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com