

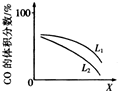
���� ��1��ͼ�������֪�ȹ���ƽ���¶ȸߣ��״����ʵ������¶�����С��˵������ƽ��������У����������ȷ�Ӧ������ӦΪ���ȷ�Ӧ��
��2��������ͼ2��3���Ȼ�ѧ����ʽ��ϸ�˹���ɼ���õ�������̼�������Ʊ��״����Ȼ�ѧ����ʽ��
����ͼ��֪��10min����ƽ�⣬ƽ��ʱ�״���Ũ�ȱ仯Ϊ0.75mol/L������v=$\frac{��c}{��t}$����v��CH3OH��������v��H2��=3v��CH3OH����
��3������ͼ���֪����n��O2��/n��CH3OH��=0.25ʱ��CH3OH��O2��������Ҫ��ӦΪ�״��Ĵ��������ɼ�ȩ�����Ʊ�H2ʱ��ÿ���n��O2��/n��CH3OH��=0.5ʱ����������࣮
��� �⣺��1����CO�ϳɼ״��ķ�ӦΪ��CO��g��+2H2��g��?CH3OH��g��������ͼ1��ʾ���¶����ߣ��״������ʵ�����С��ƽ�������ƶ���������Ӧ���ȣ��������Ӧ�ġ�H��0��
�ʴ�Ϊ������
��2����ͼ2��3���Ȼ�ѧ����ʽ��CO��g��+H2O��l��=CO2��g��+H2��g����H=-41KJ/mol��
��CO��g��+2H2��g��=CH3OH��l����H=-��510-419��KJ/mol=-91KJ/mol��
�ɸ�˹���ɢ�-�ٵõ�������̼�������Ʊ��״����Ȼ�ѧ����ʽ��CO2��g��+3H2��g��=CH3OH��l��+H2O��l����H=-50KJ/mol��
�ʴ�Ϊ��CO2��g��+3H2��g��=CH3OH��l��+H2O��l����H=-50KJ/mol��
��10min����ƽ�⣬ƽ��ʱ�״���Ũ�ȱ仯Ϊ0.75mol/L���ӷ�Ӧ��ʼ��ƽ�⣬�״���ƽ����Ӧ����v��CH3OH��=$\frac{0.75mol/L}{10min}$=0.075mol/��L•min����
v��H2��=3v��CH3OH��=3��0.075mol/��L•min��=0.225mol/��L•min����
�ʴ�Ϊ��0.225 mol/��L��min����
��3������ͼ���֪����n��O2��/n��CH3OH��=0.25ʱ��CH3OH��O2��������Ҫ��ӦΪ�״��Ĵ��������ɼ�ȩ����Ӧ�ķ���ʽΪ��2CH3OH+O2?2HCHO+2H2O������ͼ����������Ʊ�H2ʱ��ÿ���n��O2��/n��CH3OH��=0.5ʱ����������࣬
�ʴ�Ϊ��0.5��
���� ���⿼�����Ȼ�ѧ����ʽ��д����ѧƽ�ⳣ�����ƶ���ת���ʡ���Ӧ���ʵ����⣬Ҫע��ͼ����ȷ��������Ŀ�Ѷ��еȣ�


 ��һ����ͬ���ɽ�����ϵ�д�
��һ����ͬ���ɽ�����ϵ�д� ������Ӧ���ϵ�д�
������Ӧ���ϵ�д� ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ϩ������̼̼˫��������ʹ���CCl4��Һ��ɫ | |
| B�� | ���� ����һ�ȴ�����һ�֣������һ�ȴ���Ҳ��һ�� ����һ�ȴ�����һ�֣������һ�ȴ���Ҳ��һ�� | |
| C�� | �Ҵ�����ϩ���������Ը�����ط�����Ӧ | |
| D�� | ���Ǻ���ѿ�ǻ�Ϊͬ���칹�壬��ά�غ͵���Ҳ��Ϊͬ���칹�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ҵ�Ͽ������÷�ӦI��ʾԭ��������Ⱦ������ŷţ�������ȼú�����е���
��ҵ�Ͽ������÷�ӦI��ʾԭ��������Ⱦ������ŷţ�������ȼú�����е���| ʱ��/min ���ʵ���/mol ���� | 0 | 2 | 4 | 6 | 8 | 10 |
| CO��g�� | 0.100 | 0.070 | 0.050 | 0.044 | 0.040 | 0.040 |
| SO2��g�� | 0.120 | 0.105 | 0.095 | 0.092 | 0.090 | 0.090 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ��֪2C��s��+2O2��g���T2CO2��g������H1��2C��s��+O2��g��=2CO��g������H2�����H1����H2 | |
| B�� | 500�桢30MPa�£���0.5mol N2��1.5molH2�����ܱյ������г�ַ�Ӧ����NH3��g��������19.3kJ�����Ȼ�ѧ����ʽΪ�� N2��g��+3H2��g��?$?_{500�棬30MPa}^{����}$2MH3��g������H=-38.6kJ•mol-1 | |
| C�� | ��20.0g NaOH��ϡ��Һ��ϡ������ȫ�кͣ��ų�28.7 kJ����������÷�Ӧ���Ȼ�ѧ����ʽΪ��NaOH��aq��+HCl��aq���TNaCl��aq��+H2O��aq������H=-57.4 kJ/mol | |
| D�� | ��֪2H2��g��+O2��g���T2H2O��g������H=-483.6 kJ/mol����������ȼ����Ϊ241.8 kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | C8H18��l��+$\frac{25}{2}$O2��g���T8CO2��g��+9H2O��l����H=-48.40kJ•mol-1 | |
| B�� | C8H18��l��+$\frac{25}{2}$O2��g���T8CO2 ��g��+9H2O��l����H=-5517.60kJ•mol-1 | |
| C�� | C8H18��l��+$\frac{25}{2}$O2��g���T8CO2��g��+9H2O��g����H=-5517.60kJ•mol-1 | |
| D�� | 2C8H18��l��+25O2��g���T16CO2 ��g��+18H2O��l����H=-11035.20kJ•mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ������ͼװ����ȡ���е����ָ�����������壨ͼ������̨�����С����ȼ������ռ�װ�þ�����ȥ����Ҫʱ���Լ��ȣ�a��b��c��d��ʾ��Ӧ�����м�����Լ��������п��Եõ����﴿���������ѡ���ǣ�������
������ͼװ����ȡ���е����ָ�����������壨ͼ������̨�����С����ȼ������ռ�װ�þ�����ȥ����Ҫʱ���Լ��ȣ�a��b��c��d��ʾ��Ӧ�����м�����Լ��������п��Եõ����﴿���������ѡ���ǣ�������| ѡ�� | ���� | a | b | c | d |
| A | CO2 | ���� | CaCO3 | ����Na2CO3��Һ | Ũ���� |
| B | NH3 | ����NH4Cl��Һ | ��ʯ�� | H2O | ����NaOH |
| C | Cl2 | Ũ���� | MnO2 | ����NaCl��Һ | Ũ���� |
| D | NO2 | ŨHNO3 | ͭм | H2O | ����NaOH |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | $\frac{{W}_{1}+{W}_{2}}{2}$ | B�� | ��$\frac{{W}_{1}+{W}_{2}}{2}$ | C�� | ��$\frac{{W}_{1}+{W}_{2}}{2}$ | D�� | ��ȷ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com