
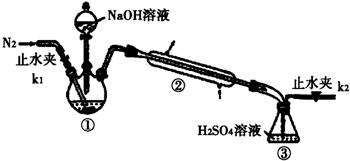
���� ��1��������������ƽ�����������þƾ��Ƽ�����100��ʧȥ�ᾧˮ��Ȼ���ڸ���������ȴ��
��2��ʵ����Ŀ���Dzⶨ笠����ӵĺ�����笠����Ӻ������ݰ��������ⶨ��������ȫ���ϳ���ͨ�뵪����
��3����Һ�д�������泥����������ữ�����ᱵ��Һ�������һ��ϴ��Һ���Ƿ������������ӣ����жϳ����Ƿ�ϴ����
��4��7.84gdz���̾��壬���Ⱥ��أ�����Ϊ5.68g���������������ˮ�������������ʵ��������ݷ�Ӧʽ2NaOH+H2SO4=Na2SO4+2H2O���ʣ����������ʵ��������ݷ�Ӧʽ2NH3+H2SO4=��NH4��2SO4����백����Ӧ����������ʵ������ݴ���������ᷴӦ�İ��������ʵ�����2Fe2++H2O2+2H+=2Fe3++2H2O�����������Ϊ1.6gΪ�����������������������ݵó�n��NH4+����n��Fe2+����n����SO42-����n��H2O��=2��1��2��6���ݴ������dz���̾���Ļ�ѧʽ���������Ӻ�笠����Ӻ���������Ӧ�����������������ݴ���д���ӷ�Ӧ����ʽ��
��� �⣺��1���ᾧˮ�IJⶨ��������Ҫ������ƽ��G����������Ҫ�ƾ��ƣ�E����������C��������̨����Ȧ��B����������������ʱ���������F���ȣ��������ձ���A����������D����
�ʴ�Ϊ��AD��
��2��ʵ����Ŀ���Dzⶨ笠����ӵĺ�����ͨ��笠����ӺͼӦ���ɵİ��������ⶨ��NH4++OH-$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O�����ݽ�����ȫ���ϳ���װ�â��б��������գ���С�����ͨ�뵪����Ϊ��֤ʵ��ȷʵ�����ǰ��Ҫ���װ�������ԣ�
�ʴ�Ϊ������Һ�еİ�ȫ���ϳ���װ�â��б��������գ����װ�������ԣ�
��3����Һ�д�������泥���δϴ��������1-2��ϡ���ᣬ�ٵ������ᱵ��Һ���а�ɫ�������ɣ����Լ�������Ƿ�ϴ���ķ����ǣ�ȡ���һ��ϴ��Һ���Թ��У�����1-2��ϡ���ᣬ�ٵ������ᱵ��Һ�����ް�ɫ�������ɣ���˵�������Ѿ�ϴ����
�ʴ�Ϊ��ȡ���һ��ϴ��Һ���Թ��У�����1-2��ϡ���ᣬ�ٵ������ᱵ��Һ�����ް�ɫ�������ɣ���˵�������Ѿ�ϴ����
��4����������ݿ�֪7.84gĦ������m��H2O��=7.84g-5.68g=2.16g��n��H2O��=$\frac{2.16g}{18g/mol}$=0.12mol�������������Ƶ����ʵ�����n=cv=lmol•L-1��40.00mL=0.04mol������ݷ�Ӧʽ2NaOH+H2SO4=Na2SO4+2H2O��֪��ʣ����������ʵ�����2mol•L-1��20.00mL��$\frac{1}{2}$=0.02mol������ݷ�Ӧʽ2NH3+H2SO4=��NH4��2SO4��֪���백����Ӧ����������ʵ�����0.04mol-0.02mol=0.02mol���������ᷴӦ�İ��������ʵ�����0.02mol��2=0.04mol�����ɵ�m��NH3��=0.68g��2Fe2++H2O2+2H+=2Fe3++2H2O�����������Ϊ1.6gΪ��������m��Fe2O3��=1.6g��n��Fe2O3��=$\frac{1.6g}{160g/mol}$=0.01mol����m��NH4+��=0.04mol��18g/mol=0.72g��m��Fe2+��=0.02mol��56g/mol=1.12g����m��SO42-��=7.84g-2.16g-0.72g-1.12g=3.84g��n��SO42-��=$\frac{3.84g}{96g/mol}$=0.04mol������n��NH4+����n��Fe2+����n����SO42-����n��H2O��=0.04mol��0.02mol��0.04mol��0.12mol=2��1��2��6����dz���̾���Ļ�ѧʽΪFeSO4•��NH4��2 SO4•6H2O ��NH4��2Fe��SO4��2•6H2O������ƿ���������Ӻ�笠����Ӻ���������Ӧ������������������ӦΪ��2NH4++Fe2++4OH-=Fe��OH��2��+2NH3��+2H2O��
�ʴ�Ϊ��FeSO4•��NH4��2 SO4•6H2O ��NH4��2Fe��SO4��2•6H2O��2NH4++Fe2++4OH-=Fe��OH��2��+2NH3��+2H2O��
���� ������Ҫ�����˾�����ɵIJⶨ��ͬʱ������ʵ��֪ʶ����Ҫ��ʵ����̷����жϣ����������IJⶨ����������ʵ�����������������ɵIJⶨ������Ū��ʵ��ԭ���ǽ���Ĺؼ�����Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��̬�⻯����ȶ���ǿ����HF��HCl��HBr��HI | |
| B�� | ԭ�Ӱ뾶��С��Mg��S��O | |
| C�� | ����ǿ����NaOH��Mg��OH��2 | |
| D�� | ������ǿ����K��Na |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
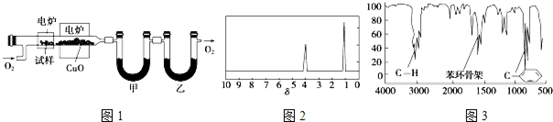
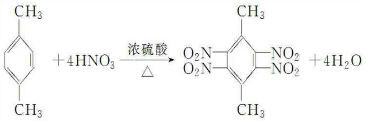 ��
�� ��
�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����£����ȳʺ�ɫ����Һ�У�Na+��NH4+��SO42-��CH3COO- | |
| B�� | �����̪��Һ�Ժ�ɫ����Һ��Na+��Ba2+��NO3-��Cl- | |
| C�� | ��ˮ�������c��H+��=1��10-13mol/L����Һ��NH4+��K+��CO32-��Cl- | |
| D�� | ���д���HCO3-����Һ��K+��Al3+��Cl-��Na+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڻ�̬�����ԭ���У�p�����������һ������s����������� | |
| B�� | ��̬Feԭ�ӵ���Χ�����Ų�ͼΪ 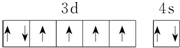 | |
| C�� | ����Ԫ�ص縺�Աȵ�Ԫ�ش���ԭ�ӵ�һ�����ܱȵ�ԭ�ӵ�һ�����ܴ� | |
| D�� | ����ԭ�Ӻ�������Ų����ص㣬��FeԪ�����ڱ���λ��ds�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢ� | B�� | �ڢۢ� | C�� | �ۢܢ� | D�� | �ڢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ۢ� | B�� | �ڢܢ� | C�� | �ڢۢܢ� | D�� | �٢ڢۢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ʯ��SiO2 | B�� | CO2��SiO2 | C�� | NaCl�� HCl | D�� | �ƺ�KCl |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com