
 ČēĶ¼ĖłŹ¾µÄ×°ÖĆ£¬½ÓĶصēŌ“5minŗ󣬵ē¼«3µÄÖŹĮæŌö¼ÓĮĖ0.64g£®
ČēĶ¼ĖłŹ¾µÄ×°ÖĆ£¬½ÓĶصēŌ“5minŗ󣬵ē¼«3µÄÖŹĮæŌö¼ÓĮĖ0.64g£®·ÖĪö ½ÓĶصēŌ“5minŗ󣬵ē¼«3µÄÖŹĮæŌö¼ÓĮĖ0.64g£¬ĖłŅŌµē¼«3ŹĒŅõ¼«£¬4ŹĒŃō¼«£¬5ŹĒŅõ¼«£¬6ŹĒŃō¼«£¬BŹĒÕż¼«£¬AŹĒøŗ¼«£¬1ŹĒŅõ¼«£¬2ŹĒŃō¼«£¬øł¾Żµē¼«·“Ó¦Ź½½įŗĻµē×ÓŹŲŗć½ųŠŠ¼ĘĖć£®
£Ø1£©AŹĒøŗ¼«£¬1ŹĒŅõ¼«£¬·¢Éś»¹Ō·“Ó¦£¬6ŹĒŃō¼«£¬CuŹĒ»īĘƵē¼«£¬µē¼«±¾ÉķŹ§µē×Ó£»
£Ø2£©µē¼«3µÄÖŹĮæŌö¼ÓĮĖ0.64g£¬¾Ż“Ė¼ĘĖćĶĄė×ÓĪļÖŹµÄĮæ²¢¼ĘĖćÅØ¶Č£»
£Ø3£©øł¾Żµē×Ó×ŖŅĘ¼ĘĖćµē½āÉś³ÉµÄĒāŃõ»ÆÄʵÄÅØ¶Č£¬½ų¶ų¼ĘĖćpH£»
£Ø4£©±ū×°ÖĆŃō¼«ÉĻŹĒĶŹ§µē×ӵƵ½ĶĄė×ӵķ“Ó¦£¬ĶĄė×Ó½ųČėµē½āÖŹ£¬øł¾Ż¾ĶĄė×ӵķŵēĖ³ŠņČ·¶Øµē¼«·“Ó¦£®
½ā“š ½ā£ŗ½ÓĶصēŌ“5minŗ󣬵ē¼«3µÄÖŹĮæŌö¼ÓĮĖ0.64g£¬ĖłŅŌµē¼«3ŹĒŅõ¼«£¬4ŹĒŃō¼«£¬5ŹĒŅõ¼«£¬6ŹĒŃō¼«£¬BŹĒÕż¼«£¬AŹĒøŗ¼«£¬1ŹĒŅõ¼«£¬2ŹĒŃō¼«£¬
£Ø1£©AŹĒøŗ¼«£¬±ū×°ÖĆŹĒµē½ā³Ų£¬1ŹĒŅõ¼«£¬µē¼«·“Ó¦Ź½ĪŖ£ŗ2H++2e-=H2”ü£¬6ŹĒŃō¼«£¬CuŹĒ»īĘƵē¼«£¬µē¼«·“Ó¦Ź½ĪŖ£ŗCu-2e-=Cu2+£®
¹Ź“š°øĪŖ£ŗøŗ£»µē½ā£»2H++2e-=H2”ü£»Cu-2e-=Cu2+£®
£Ø2£©µē¼«3µÄÖŹĮæŌö¼ÓĮĖ0.64g£¬ĖłŅŌµē¼«3ŹĒŅõ¼«£¬Éś³É½šŹōĶŹĒ0.01mol£¬ĖłŅŌĶĄė×ÓµÄĪļÖŹµÄĮæŹĒ0.01mol£¬ÅØ¶ČŹĒ£ŗ$\frac{0.01mol}{0.1L}$=0.1mol/L£¬¹Ź“š°øĪŖ£ŗ0.1mol/L£»
£Ø3£©ČōAÖŠČÜŅŗµÄĢå»żŹĒ100mL£¬ĶØČėÖ±Į÷µē5minŹ±£¬ÓÉ£Ø2£©¼ĘĖćæÉÖŖÉś³É½šŹōĶŹĒ0.01mol£¬×ŖŅʵē×Ó0.02mol£¬Éś³Én£ØOH-£©=0.02mol£¬µē½āŗó£¬ČÜŅŗµÄc£ØOH-£©=$\frac{0.02mol}{0.1L}$=0.2mol/L£¬ĖłŅŌpH=14-lg5=13.3£¬¹Ź“š°øĪŖ£ŗ13.3£»
£Ø4£©±ū×°ÖĆŃō¼«ÉĻŹĒĶŹ§µē×ӵƵ½ĶĄė×ӵķ“Ó¦£¬ĶĄė×Ó½ųČėµē½āÖŹ£¬µ±ŅųĄė×ӵƵē×ÓĶź±Ļ£¬ĶĄė×Ó»¹æÉŅŌµĆµ½µē×Ó£¬¼ĢŠų·“Ó¦£¬ĖłŅŌ±ū×°ÖĆÖŠ²»ŌŁĪö³öAg²»ÄÜĖµĆ÷µē½ā½įŹų£¬¹Ź“š°øĪŖ£ŗ“ķĪó£»±ū×°ÖĆŃō¼«ÉĻŹĒĶŹ§µē×ӵƵ½ĶĄė×ӵķ“Ó¦£¬ĶĄė×Ó½ųČėµē½āÖŹ£¬µ±ŅųĄė×ӵƵē×ÓĶź±Ļ£¬ĶĄė×Ó»¹æÉŅŌµĆµ½µē×Ó£¬¼ĢŠų·“Ó¦£®
µćĘĄ ±¾Ģāæ¼²éѧɜµē½ā³ŲµÄ¹¤×÷ŌĄķŅŌ¼°µē¼«·“Ó¦Ź½µÄŹéŠ“ŗĶµē×ÓŹŲŗćµÄ¼ĘĖćÖŖŹ¶£¬×¢ŅāÖŖŹ¶µÄ¹éÄÉŗĶŹįĄķŹĒ¹Ų¼ü£¬ÄѶČÖŠµČ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
| ŹµŃ鱹ŗÅ | HAĪļÖŹµÄĮæ ÅØ¶Č£Ømol/L£© | NaOHĪļÖŹµÄ ĮæÅØ¶Č£Ømol/L£© | »ģŗĻČÜŅŗµÄpH |
| ¼× | 0.2 | 0.2 | pH=a |
| ŅŅ | c1 | 0.2 | pH=7 |
| ±ū | 0.2 | 0.1 | pH£¾7 |
| ¶” | 0.1 | 0.1 | pH=9 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
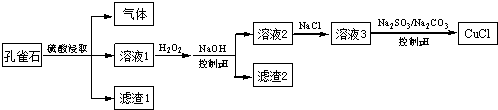
| A£® | H2O2½«ČÜŅŗ1ÖŠFe2+Ńõ»ÆĪŖFe3+£¬ŌŁĶعżæŲÖĘpH×Ŗ»ÆĪŖFe£ØOH£©3³żČ„ | |
| B£® | SO32-½«ČÜŅŗ3ÖŠµÄCu2+»¹Ō£¬·“Ó¦µĆµ½CuCl | |
| C£® | CO32-×÷ÓĆŹĒæŲÖĘČÜŅŗpH£¬“ŁŹ¹CuCl³ĮµķµÄÉś³É | |
| D£® | ČōøıäŹŌ¼Į¼ÓČėĖ³Šņ£¬½«ČÜŅŗ3»ŗĀż¼ÓČėµ½ŗ¬“óĮæSO32-/CO32-µÄČÜŅŗÖŠ£¬Ķ¬ŃłæÉÖĘČ”CuCl |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | NH4Cl | B£® | NH3•H2O | C£® | Cu | D£® | CH3CH2OH |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
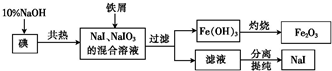
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | NaCl | B£® | AlCl3 | C£® | CaCl2 | D£® | FeCl3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| ²Ł×÷ŗĶĻÖĻó | ½įĀŪ | |
| A | Č”Ä³ČÜŅŗÉŁŠķ£¬¼ÓČėĖį»ÆµÄBa£ØNO3£©2ČÜŅŗ£¬²śÉś°×É«³Įµķ | øĆČÜŅŗŅ»¶Øŗ¬SO42- |
| B | NaHCO3ČÜŅŗÓėNaAlO2ČÜŅŗ»ģŗĻ²śÉś°×É«³Įµķ | ½įŗĻH+µÄÄÜĮ¦£ŗCO32-£¼AlO2- |
| C | ijČÜŅŗ¼ÓČėŃĪĖį²śÉśŹ¹ŹÆ»ŅĖ®±ä»ė×ĒµÄĪŽÉ«ĪŽĪ¶ĘųĢå | øĆČÜŅŗæĻ¶Øŗ¬HCO3-”¢CO32-ÖŠµÄŅ»ÖÖ»ņ¶žÖÖ |
| D | ²ā¶ØµČÅØ¶ČµÄNa2CO3ŗĶNa2SO3ČÜŅŗµÄpH£»Ē°ÕßpH±ČŗóÕßµÄ“ó£» | ·Ē½šŹōŠŌ£ŗS£¾C |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com